Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization
- PMID: 32821743
- PMCID: PMC7427034
- DOI: 10.1093/burnst/tkaa028
Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization
Abstract
Background: Autologous platelet-rich plasma (PRP) has been suggested to be effective for wound healing. However, evidence for its use in patients with acute and chronic wounds remains insufficient. The aims of this study were to comprehensively examine the effectiveness, synergy and possible mechanism of PRP-mediated improvement of acute skin wound repair.
Methods: Full-thickness wounds were made on the back of C57/BL6 mice. PRP or saline solution as a control was administered to the wound area. Wound healing rate, local inflammation, angiogenesis, re-epithelialization and collagen deposition were measured at days 3, 5, 7 and 14 after skin injury. The biological character of epidermal stem cells (ESCs), which reflect the potential for re-epithelialization, was further evaluated in vitro and in vivo.
Results: PRP strongly improved skin wound healing, which was associated with regulation of local inflammation, enhancement of angiogenesis and re-epithelialization. PRP treatment significantly reduced the production of inflammatory cytokines interleukin-17A and interleukin-1β. An increase in the local vessel intensity and enhancement of re-epithelialization were also observed in animals with PRP administration and were associated with enhanced secretion of growth factors such as vascular endothelial growth factor and insulin-like growth factor-1. Moreover, PRP treatment ameliorated the survival and activated the migration and proliferation of primary cultured ESCs, and these effects were accompanied by the differentiation of ESCs into adult cells following the changes of CD49f and keratin 10 and keratin 14.
Conclusion: PRP improved skin wound healing by modulating inflammation and increasing angiogenesis and re-epithelialization. However, the underlying regulatory mechanism needs to be investigated in the future. Our data provide a preliminary theoretical foundation for the clinical administration of PRP in wound healing and skin regeneration.
Keywords: Angiogenesis; Collagen deposition; Epidermal stem cells; Inflammation; Re-epithelialization; Wound healing; platelet-rich plasma.
© The Author(s) 2020. Published by Oxford University Press.
Figures
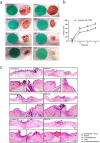
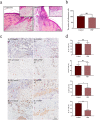
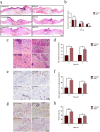
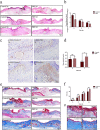
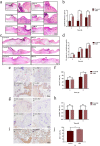
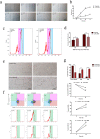
Similar articles
-
[Effects of human adipose-derived mesenchymal stem cells and platelet-rich plasma on healing of wounds with full-thickness skin defects in mice].Zhonghua Shao Shang Za Zhi. 2018 Dec 20;34(12):887-894. doi: 10.3760/cma.j.issn.1009-2587.2018.12.013. Zhonghua Shao Shang Za Zhi. 2018. PMID: 30585053 Chinese.
-
[Exploration of the mechanism of human platelet-rich plasma in regulating and controlling human epidermal stem cells for promoting wound re-epithelialization at transcriptome level].Zhonghua Shao Shang Za Zhi. 2020 Oct 20;36(10):915-922. doi: 10.3760/cma.j.cn501120-20200707-00341. Zhonghua Shao Shang Za Zhi. 2020. PMID: 33105943 Chinese.
-
Co-administration of platelet-rich plasma and small intestinal submucosa is more beneficial than their individual use in promoting acute skin wound healing.Burns Trauma. 2021 Nov 30;9:tkab033. doi: 10.1093/burnst/tkab033. eCollection 2021. Burns Trauma. 2021. PMID: 35464804 Free PMC article.
-
Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization.Stem Cell Res Ther. 2020 Jun 11;11(1):232. doi: 10.1186/s13287-020-01755-y. Stem Cell Res Ther. 2020. PMID: 32527289 Free PMC article. Review.
-
The role of allogeneic platelet-rich plasma in patients with diabetic foot ulcer: Current perspectives and future challenges.Front Bioeng Biotechnol. 2022 Sep 29;10:993436. doi: 10.3389/fbioe.2022.993436. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36246379 Free PMC article. Review.
Cited by
-
Platelet-rich plasma ameliorates lipopolysaccharide-induced cardiac injury by inflammation and ferroptosis regulation.Front Pharmacol. 2022 Oct 18;13:1026641. doi: 10.3389/fphar.2022.1026641. eCollection 2022. Front Pharmacol. 2022. PMID: 36330090 Free PMC article.
-
Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts.Int J Mol Sci. 2021 Nov 23;22(23):12623. doi: 10.3390/ijms222312623. Int J Mol Sci. 2021. PMID: 34884429 Free PMC article.
-
Comparison of the short-term effect of intra-articular hyaluronic acid and platelet-rich plasma injections in knee osteoarthritis: a randomized clinical trial.J Prev Med Hyg. 2024 Aug 31;65(2):E214-E220. doi: 10.15167/2421-4248/jpmh2024.65.2.3270. eCollection 2024 Jun. J Prev Med Hyg. 2024. PMID: 39430992 Free PMC article. Clinical Trial.
-
Comparative effectiveness of intra-articular therapies in knee osteoarthritis: a meta-analysis comparing platelet-rich plasma (PRP) with other treatment modalities.Ann Med Surg (Lond). 2023 Dec 15;86(1):361-372. doi: 10.1097/MS9.0000000000001615. eCollection 2024 Jan. Ann Med Surg (Lond). 2023. PMID: 38222750 Free PMC article. Review.
-
Recombinant human epidermal growth factor combined with vacuum sealing drainage for wound healing in Bama pigs.Mil Med Res. 2021 Mar 9;8(1):18. doi: 10.1186/s40779-021-00308-5. Mil Med Res. 2021. PMID: 33685528 Free PMC article.
References
-
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004; 62(4): 489–96. - PubMed
-
- Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004; 114(6): 1502–8. - PubMed
-
- Sun Y, Cao Y, Zhao R, Xu F, Wu D, Wang Y. The role of autologous PRP on deep partial-thickness burn wound healing in Bama pigs. J Burn Care Res. 2020; 41(3): 657–62. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

