BTN3A1 governs antitumor responses by coordinating αβ and γδ T cells
- PMID: 32820120
- PMCID: PMC7646930
- DOI: 10.1126/science.aay2767
BTN3A1 governs antitumor responses by coordinating αβ and γδ T cells
Abstract
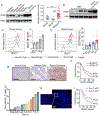
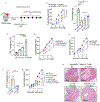
Gamma delta (γδ) T cells infiltrate most human tumors, but current immunotherapies fail to exploit their in situ major histocompatibility complex-independent tumoricidal potential. Activation of γδ T cells can be elicited by butyrophilin and butyrophilin-like molecules that are structurally similar to the immunosuppressive B7 family members, yet how they regulate and coordinate αβ and γδ T cell responses remains unknown. Here, we report that the butyrophilin BTN3A1 inhibits tumor-reactive αβ T cell receptor activation by preventing segregation of N-glycosylated CD45 from the immune synapse. Notably, CD277-specific antibodies elicit coordinated restoration of αβ T cell effector activity and BTN2A1-dependent γδ lymphocyte cytotoxicity against BTN3A1+ cancer cells, abrogating malignant progression. Targeting BTN3A1 therefore orchestrates cooperative killing of established tumors by αβ and γδ T cells and may present a treatment strategy for tumors resistant to existing immunotherapies.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



Similar articles
-
EBV latent membrane protein 1 augments γδ T cell cytotoxicity against nasopharyngeal carcinoma by induction of butyrophilin molecules.Theranostics. 2023 Jan 1;13(2):458-471. doi: 10.7150/thno.78395. eCollection 2023. Theranostics. 2023. PMID: 36632221 Free PMC article.
-
Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells.Science. 2020 Feb 7;367(6478):eaay5516. doi: 10.1126/science.aay5516. Epub 2020 Jan 9. Science. 2020. PMID: 31919129
-
Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology.Proc Natl Acad Sci U S A. 2018 Jan 30;115(5):1039-1044. doi: 10.1073/pnas.1701237115. Epub 2018 Jan 16. Proc Natl Acad Sci U S A. 2018. PMID: 29339503 Free PMC article.
-
γδ T Cells in the Tumor Microenvironment-Interactions With Other Immune Cells.Front Immunol. 2022 Jul 11;13:894315. doi: 10.3389/fimmu.2022.894315. eCollection 2022. Front Immunol. 2022. PMID: 35880177 Free PMC article. Review.
-
Regulation of Immunity by Butyrophilins.Annu Rev Immunol. 2016 May 20;34:151-72. doi: 10.1146/annurev-immunol-041015-055435. Epub 2016 Jan 11. Annu Rev Immunol. 2016. PMID: 26772212 Review.
Cited by
-
Dual targeting of cancer metabolome and stress antigens affects transcriptomic heterogeneity and efficacy of engineered T cells.Nat Immunol. 2024 Jan;25(1):88-101. doi: 10.1038/s41590-023-01665-0. Epub 2023 Nov 27. Nat Immunol. 2024. PMID: 38012415
-
BTN3A: A Promising Immune Checkpoint for Cancer Prognosis and Treatment.Int J Mol Sci. 2022 Nov 3;23(21):13424. doi: 10.3390/ijms232113424. Int J Mol Sci. 2022. PMID: 36362212 Free PMC article. Review.
-
Synthesis and Metabolism of BTN3A1 Ligands: Studies on Diene Modifications to the Phosphoantigen Scaffold.ACS Med Chem Lett. 2022 Jan 27;13(2):164-170. doi: 10.1021/acsmedchemlett.1c00408. eCollection 2022 Feb 10. ACS Med Chem Lett. 2022. PMID: 35178171 Free PMC article.
-
Gamma delta TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds.J Immunother Cancer. 2021 Nov;9(11):e003850. doi: 10.1136/jitc-2021-003850. J Immunother Cancer. 2021. PMID: 34815357 Free PMC article.
-
Cancer cell-expressed BTNL2 facilitates tumour immune escape via engagement with IL-17A-producing γδ T cells.Nat Commun. 2022 Jan 11;13(1):231. doi: 10.1038/s41467-021-27936-8. Nat Commun. 2022. PMID: 35017553 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

