KAT5 acetylates cGAS to promote innate immune response to DNA virus
- PMID: 32817552
- PMCID: PMC7474609
- DOI: 10.1073/pnas.1922330117
KAT5 acetylates cGAS to promote innate immune response to DNA virus
Abstract
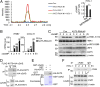
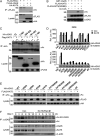
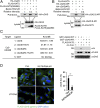
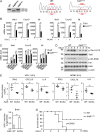
The DNA sensor cGMP-AMP synthase (cGAS) senses cytosolic microbial or self DNA to initiate a MITA/STING-dependent innate immune response. cGAS is regulated by various posttranslational modifications at its C-terminal catalytic domain. Whether and how its N-terminal unstructured domain is regulated by posttranslational modifications remain unknown. We identified the acetyltransferase KAT5 as a positive regulator of cGAS-mediated innate immune signaling. Overexpression of KAT5 potentiated viral-DNA-triggered transcription of downstream antiviral genes, whereas a KAT5 deficiency had the opposite effects. Mice with inactivated Kat5 exhibited lower levels of serum cytokines in response to DNA virus infection, higher viral titers in the brains, and more susceptibility to DNA-virus-induced death. Mechanistically, KAT5 catalyzed acetylation of cGAS at multiple lysine residues in its N-terminal domain, which promoted its DNA-binding ability. Our findings suggest that KAT5-mediated cGAS acetylation at its N terminus is important for efficient innate immune response to DNA virus.
Keywords: DNA virus; KAT5; acetylation; cGAS; innate immune response.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response.Nat Commun. 2018 Aug 22;9(1):3349. doi: 10.1038/s41467-018-05559-w. Nat Commun. 2018. PMID: 30135424 Free PMC article.
-
The DNA Sensor cGAS is Decorated by Acetylation and Phosphorylation Modifications in the Context of Immune Signaling.Mol Cell Proteomics. 2020 Jul;19(7):1193-1208. doi: 10.1074/mcp.RA120.001981. Epub 2020 Apr 28. Mol Cell Proteomics. 2020. PMID: 32345711 Free PMC article.
-
Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity.Nat Immunol. 2016 Apr;17(4):369-78. doi: 10.1038/ni.3356. Epub 2016 Feb 1. Nat Immunol. 2016. PMID: 26829768
-
Delicate regulation of the cGAS-MITA-mediated innate immune response.Cell Mol Immunol. 2018 Jul;15(7):666-675. doi: 10.1038/cmi.2016.51. Epub 2018 Feb 19. Cell Mol Immunol. 2018. PMID: 29456253 Free PMC article. Review.
-
Function and Regulation of Nuclear DNA Sensors During Viral Infection and Tumorigenesis.Front Immunol. 2021 Jan 11;11:624556. doi: 10.3389/fimmu.2020.624556. eCollection 2020. Front Immunol. 2021. PMID: 33505405 Free PMC article. Review.
Cited by
-
mTORC1 activity regulates post-translational modifications of glycine decarboxylase to modulate glycine metabolism and tumorigenesis.Nat Commun. 2021 Jul 9;12(1):4227. doi: 10.1038/s41467-021-24321-3. Nat Commun. 2021. PMID: 34244482 Free PMC article.
-
Unconventional posttranslational modification in innate immunity.Cell Mol Life Sci. 2024 Jul 6;81(1):290. doi: 10.1007/s00018-024-05319-8. Cell Mol Life Sci. 2024. PMID: 38970666 Free PMC article. Review.
-
Role of Post-Translational Modifications of cGAS in Innate Immunity.Int J Mol Sci. 2020 Oct 22;21(21):7842. doi: 10.3390/ijms21217842. Int J Mol Sci. 2020. PMID: 33105828 Free PMC article. Review.
-
Post-translational modification control of viral DNA sensors and innate immune signaling.Adv Virus Res. 2021;109:163-199. doi: 10.1016/bs.aivir.2021.03.001. Epub 2021 Apr 16. Adv Virus Res. 2021. PMID: 33934827 Free PMC article.
-
Control of innate immunity by the cGAS-STING pathway.Immunol Cell Biol. 2022 Jul;100(6):409-423. doi: 10.1111/imcb.12555. Epub 2022 May 25. Immunol Cell Biol. 2022. PMID: 35485309 Free PMC article. Review.
References
-
- Akira S., Uematsu S., Takeuchi O., Pathogen recognition and innate immunity. Cell 124, 783–801 (2006). - PubMed
-
- Hu M. M., Shu H. B., Cytoplasmic mechanisms of recognition and defense of microbial nucleic acids. Annu. Rev. Cell Dev. Biol. 34, 357–379 (2018). - PubMed
-
- Wu J., Chen Z. J., Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32, 461–488 (2014). - PubMed
-
- Barbalat R., Ewald S. E., Mouchess M. L., Barton G. M., Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 29, 185–214 (2011). - PubMed
-
- Takeuchi O., Akira S., Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

