Core Outcomes Set for Trials in People With Coronavirus Disease 2019
- PMID: 32804792
- PMCID: PMC7448717
- DOI: 10.1097/CCM.0000000000004585
Core Outcomes Set for Trials in People With Coronavirus Disease 2019
Abstract
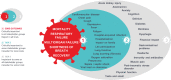
Objectives: The outcomes reported in trials in coronavirus disease 2019 are extremely heterogeneous and of uncertain patient relevance, limiting their applicability for clinical decision-making. The aim of this workshop was to establish a core outcomes set for trials in people with suspected or confirmed coronavirus disease 2019.
Design: Four international online multistakeholder consensus workshops were convened to discuss proposed core outcomes for trials in people with suspected or confirmed coronavirus disease 2019, informed by a survey involving 9,289 respondents from 111 countries. The transcripts were analyzed thematically. The workshop recommendations were used to finalize the core outcomes set.
Setting: International.
Subjects: Adults 18 years old and over with confirmed or suspected coronavirus disease 2019, their family members, members of the general public and health professionals (including clinicians, policy makers, regulators, funders, researchers).
Interventions: None.
Measurements: None.
Main results: Six themes were identified. "Responding to the critical and acute health crisis" reflected the immediate focus on saving lives and preventing life-threatening complications that underpinned the high prioritization of mortality, respiratory failure, and multiple organ failure. "Capturing different settings of care" highlighted the need to minimize the burden on hospitals and to acknowledge outcomes in community settings. "Encompassing the full trajectory and severity of disease" was addressing longer term impacts and the full spectrum of illness (e.g. shortness of breath and recovery). "Distinguishing overlap, correlation and collinearity" meant recognizing that symptoms such as shortness of breath had distinct value and minimizing overlap (e.g. lung function and pneumonia were on the continuum toward respiratory failure). "Recognizing adverse events" refers to the potential harms of new and evolving interventions. "Being cognizant of family and psychosocial wellbeing" reflected the pervasive impacts of coronavirus disease 2019.
Conclusions: Mortality, respiratory failure, multiple organ failure, shortness of breath, and recovery are critically important outcomes to be consistently reported in coronavirus disease 2019 trials.
Conflict of interest statement
Dr. Tong is supported by The University of Sydney Robinson Fellowship. Dr. Douglas is principal investigator of clinical and translational research studies of acute respiratory distress syndrome, sepsis, and coronavirus disease 2019 from the National Institutes of Health, Roche Pharmaceuticals and Genentech. Research grants are to his institution, Denver Health Medical Center. Dr. Morris is supported by a Clinical Research Career Development Fellowship from the Wellcome Trust (WT 2055214/Z/16/Z). Dr. Povoa had received lecture fees from Orion, Pfizer, and Technofage. Dr. Azevedo received funding from Halex Istar and Pfizer. Dr. Flemyng received funding from Cochrane. Dr. Mer received funding from Pfizer, MSD, Sanofi Aventis, Aspen, and Astellas. Dr. Morris' institution received funding from Wellcome Trust and he received funding from Wellcome Trust/COAF. Dr. Smyth's institution received funding from Vertex; he received funding from Vertex, Teva and Novartis; and he has a patent issued "Alkyl quinolones as biomarkers of Pseudomonas aeruginosa infection and uses thereof." Dr. Turner's institution received funding from Australian Commonwealth Government, Victorian State Government, Australian Stroke Foundation, and she has also provided technical advice to WHO, and the Australian Red Cross Lifeblood service undertaken unrelated consultancies with the Burnet Institute and USAID, and she is an investigator on unrelated living diabetes guidelines funded by several diabetes organizations in Australia. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Similar articles
-
International Survey to Establish Prioritized Outcomes for Trials in People With Coronavirus Disease 2019.Crit Care Med. 2020 Nov;48(11):1612-1621. doi: 10.1097/CCM.0000000000004584. Crit Care Med. 2020. PMID: 32804789 Free PMC article.
-
Core Outcome Measures for Trials in People With Coronavirus Disease 2019: Respiratory Failure, Multiorgan Failure, Shortness of Breath, and Recovery.Crit Care Med. 2021 Mar 1;49(3):503-516. doi: 10.1097/CCM.0000000000004817. Crit Care Med. 2021. PMID: 33400475 Free PMC article.
-
The SARS-CoV-2 Ivermectin Navarra-ISGlobal Trial (SAINT) to Evaluate the Potential of Ivermectin to Reduce COVID-19 Transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: A structured summary of a study protocol for a randomized control pilot trial.Trials. 2020 Jun 8;21(1):498. doi: 10.1186/s13063-020-04421-z. Trials. 2020. PMID: 32513289 Free PMC article.
-
Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review.JAMA. 2020 Aug 25;324(8):782-793. doi: 10.1001/jama.2020.12839. JAMA. 2020. PMID: 32648899 Review.
-
Masks for Prevention of Respiratory Virus Infections, Including SARS-CoV-2, in Health Care and Community Settings : A Living Rapid Review.Ann Intern Med. 2020 Oct 6;173(7):542-555. doi: 10.7326/M20-3213. Epub 2020 Jun 24. Ann Intern Med. 2020. Update in: Ann Intern Med. 2023 Jun;176(6):827-835. doi: 10.7326/M23-0570. PMID: 32579379 Free PMC article. Updated. Review.
Cited by
-
Study of Postacute Sequelae of COVID-19 Using Digital Wearables: Protocol for a Prospective Longitudinal Observational Study.JMIR Res Protoc. 2024 Aug 16;13:e57382. doi: 10.2196/57382. JMIR Res Protoc. 2024. PMID: 39150750 Free PMC article.
-
Hospitalization Endpoint in Clinical Trials of Outpatient Settings: using Anti-SARS-COV-2 Therapy as an Example.Clin Epidemiol. 2024 May 23;16:357-365. doi: 10.2147/CLEP.S464310. eCollection 2024. Clin Epidemiol. 2024. PMID: 38803423 Free PMC article. Review.
-
A prospective natural history study of post acute sequalae of COVID-19 using digital wearables: Study protocol.Res Sq [Preprint]. 2023 Dec 7:rs.3.rs-3694818. doi: 10.21203/rs.3.rs-3694818/v1. Res Sq. 2023. Update in: JMIR Res Protoc. 2024 Aug 16;13:e57382. doi: 10.2196/57382. PMID: 38105936 Free PMC article. Updated. Preprint.
-
Awareness and experiences on core outcome set development and use amongst stakeholders from low- and middle- income countries: An online survey.PLOS Glob Public Health. 2023 Dec 5;3(12):e0002574. doi: 10.1371/journal.pgph.0002574. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 38051748 Free PMC article.
-
Development of a core outcome set for traumatic brachial plexus injury.J Hand Surg Eur Vol. 2024 May;49(5):554-563. doi: 10.1177/17531934231212973. Epub 2023 Nov 21. J Hand Surg Eur Vol. 2024. PMID: 37987677 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources