Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors
- PMID: 32793604
- PMCID: PMC7385189
- DOI: 10.3389/fcell.2020.00672
Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors
Abstract
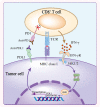
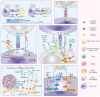
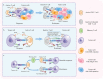
In cancer-immunity cycle, the immune checkpoint PD1 and its ligand PDL1 act as accomplices to help tumors resist to immunity-induced apoptosis and promote tumor progression. Immunotherapy targeting PD1/PDL1 axis can effectively block its pro-tumor activity. Anti-PD1/PDL1 therapy has achieved great success in the past decade. However, only a subset of patients showed clinical responses. Most of the patients can not benefit from anti-PD1/PDL1 therapy. Furthermore, a large group of responders would develop acquired resistance after initial responses. Therefore, understanding the mechanisms of resistance is necessary for improving anti-PD1/PDL1 efficacy. Currently, researchers have identified primary resistance mechanisms which include insufficient tumor immunogenicity, disfunction of MHCs, irreversible T cell exhaustion, primary resistance to IFN-γ signaling, and immunosuppressive microenvironment. Some oncogenic signaling pathways also contribute to the primary resistance. Under the pressure applied by anti-PD1/PDL1 therapy, tumors experience immunoediting and preserve beneficial mutations, upregulate the compensatory inhibitory signaling and induce re-exhaustion of T cells, all of which may attenuate the durability of the therapy. Here we explore the underlying mechanisms in detail, review biomarkers that help identifying responders among patients and discuss the strategies that may relieve the anti-PD1/PDL1 resistance.
Keywords: PD1; PDL1; cancer; immunotherapy; mechanism; resistance.
Copyright © 2020 Lei, Wang, Sun, Wang and Zhang.
Figures
Similar articles
-
PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy.Front Pharmacol. 2021 Sep 1;12:731798. doi: 10.3389/fphar.2021.731798. eCollection 2021. Front Pharmacol. 2021. PMID: 34539412 Free PMC article. Review.
-
Expanded human NK cells from lung cancer patients sensitize patients' PDL1-negative tumors to PD1-blockade therapy.J Immunother Cancer. 2021 Jan;9(1):e001933. doi: 10.1136/jitc-2020-001933. J Immunother Cancer. 2021. PMID: 33479024 Free PMC article.
-
Integration of cancer stemness and neoantigen load to predict responsiveness to anti-PD1/PDL1 therapy.Front Cell Dev Biol. 2022 Nov 17;10:1003656. doi: 10.3389/fcell.2022.1003656. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36467413 Free PMC article.
-
Proton beam radiotherapy combined with anti-PD1/PDL1 immune checkpoint inhibitors for advanced hepatocellular carcinoma.Am J Cancer Res. 2022 Apr 15;12(4):1606-1620. eCollection 2022. Am J Cancer Res. 2022. PMID: 35530291 Free PMC article.
-
Epigenetic modifications: Critical participants of the PD‑L1 regulatory mechanism in solid tumors (Review).Int J Oncol. 2022 Nov;61(5):134. doi: 10.3892/ijo.2022.5424. Epub 2022 Sep 21. Int J Oncol. 2022. PMID: 36129152 Free PMC article. Review.
Cited by
-
Unraveling T cell exhaustion in the immune microenvironment of osteosarcoma via single-cell RNA transcriptome.Cancer Immunol Immunother. 2024 Jan 27;73(2):35. doi: 10.1007/s00262-023-03585-2. Cancer Immunol Immunother. 2024. PMID: 38280005 Free PMC article.
-
Personalized Targeted Therapeutic Strategies against Oral Squamous Cell Carcinoma. An Evidence-Based Review of Literature.Int J Nanomedicine. 2022 Sep 15;17:4293-4306. doi: 10.2147/IJN.S377816. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36134201 Free PMC article. Review.
-
Immunotherapy for colorectal cancer: insight from inherited genetics.Trends Cancer. 2024 May;10(5):444-456. doi: 10.1016/j.trecan.2024.01.008. Epub 2024 Feb 14. Trends Cancer. 2024. PMID: 38360438 Review.
-
Targeted nanomedicines remodeling immunosuppressive tumor microenvironment for enhanced cancer immunotherapy.Acta Pharm Sin B. 2022 Dec;12(12):4327-4347. doi: 10.1016/j.apsb.2022.11.001. Epub 2022 Nov 4. Acta Pharm Sin B. 2022. PMID: 36561994 Free PMC article. Review.
-
CD93 serves as a potential biomarker of gastric cancer and correlates with the tumor microenvironment.World J Clin Cases. 2023 Feb 6;11(4):738-755. doi: 10.12998/wjcc.v11.i4.738. Epub 2023 Jan 6. World J Clin Cases. 2023. PMID: 36818626 Free PMC article.
References
-
- Anagnostou V., Smith K. N., Forde P. M., Niknafs N., Bhattacharya R., White J., et al. (2017). Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 7 264–276. 10.1158/2159-8290.CD-16-0828 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

