From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine
- PMID: 32781644
- PMCID: PMC7460064
- DOI: 10.3390/md18080414
From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine
Abstract
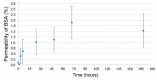
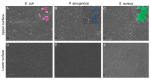
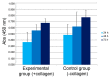
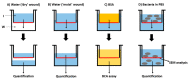
Collagen-based skin-like scaffolds (CBSS) are promising alternatives to skin grafts to repair wounds and injuries. In this work, we propose that the common marine invertebrate sea urchin represents a promising and eco-friendly source of native collagen to develop innovative CBSS for skin injury treatment. Sea urchin food waste after gonad removal was here used to extract fibrillar glycosaminoglycan (GAG)-rich collagen to produce bilayer (2D + 3D) CBSS. Microstructure, mechanical stability, permeability to water and proteins, ability to exclude bacteria and act as scaffolding for fibroblasts were evaluated. Our data show that the thin and dense 2D collagen membrane strongly reduces water evaporation (less than 5% of water passes through the membrane after 7 days) and protein diffusion (less than 2% of BSA passes after 7 days), and acts as a barrier against bacterial infiltration (more than 99% of the different tested bacterial species is retained by the 2D collagen membrane up to 48 h), thus functionally mimicking the epidermal layer. The thick sponge-like 3D collagen scaffold, structurally and functionally resembling the dermal layer, is mechanically stable in wet conditions, biocompatible in vitro (seeded fibroblasts are viable and proliferate), and efficiently acts as a scaffold for fibroblast infiltration. Thus, thanks to their chemical and biological properties, CBSS derived from sea urchins might represent a promising, eco-friendly, and economically sustainable biomaterial for tissue regenerative medicine.
Keywords: eco-friendly biomaterial; fibrillar collagen; marine collagen-based skin-like scaffolds; regenerative medicine; sea urchins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
A Prototype Skin Substitute, Made of Recycled Marine Collagen, Improves the Skin Regeneration of Sheep.Animals (Basel). 2021 Apr 23;11(5):1219. doi: 10.3390/ani11051219. Animals (Basel). 2021. PMID: 33922557 Free PMC article.
-
Marine-derived collagen biomaterials from echinoderm connective tissues.Mar Environ Res. 2017 Jul;128:46-57. doi: 10.1016/j.marenvres.2016.03.007. Epub 2016 Mar 31. Mar Environ Res. 2017. PMID: 27063846
-
2D Collagen Membranes from Marine Demosponge Chondrosia reniformis (Nardo, 1847) for Skin-Regenerative Medicine Applications: An In Vitro Evaluation.Mar Drugs. 2023 Jul 28;21(8):428. doi: 10.3390/md21080428. Mar Drugs. 2023. PMID: 37623709 Free PMC article.
-
Traditional Chinese medicine--sea urchin.Mini Rev Med Chem. 2014;14(6):537-42. doi: 10.2174/1389557514666140529224147. Mini Rev Med Chem. 2014. PMID: 24873818 Review.
-
[Clinical regenerative medicine: the skin].Nihon Rinsho. 2008 May;66(5):961-5. Nihon Rinsho. 2008. PMID: 18464517 Review. Japanese.
Cited by
-
The optimization of PLGA knitted mesh reinforced-collagen/chitosan scaffold for the healing of full-thickness skin defects.J Biomed Mater Res B Appl Biomater. 2023 Apr;111(4):763-774. doi: 10.1002/jbm.b.35187. Epub 2022 Nov 11. J Biomed Mater Res B Appl Biomater. 2023. PMID: 36367718 Free PMC article.
-
Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo.Mar Drugs. 2023 Jan 19;21(2):59. doi: 10.3390/md21020059. Mar Drugs. 2023. PMID: 36827101 Free PMC article.
-
Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies.Life (Basel). 2021 Jul 7;11(7):665. doi: 10.3390/life11070665. Life (Basel). 2021. PMID: 34357037 Free PMC article. Review.
-
Mutable Collagenous Tissue: A Concept Generator for Biomimetic Materials and Devices.Mar Drugs. 2024 Jan 7;22(1):37. doi: 10.3390/md22010037. Mar Drugs. 2024. PMID: 38248662 Free PMC article. Review.
-
Novel Electrospun Polycaprolactone/Calcium Alginate Scaffolds for Skin Tissue Engineering.Materials (Basel). 2022 Dec 23;16(1):136. doi: 10.3390/ma16010136. Materials (Basel). 2022. PMID: 36614475 Free PMC article.
References
-
- Crawford M.E. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery, In Autografts, Allografts and Xenografts in Cutaneous Surgery. 2nd ed. Volume 20. Saunders Ltd.; Nottingham, UK: 2012. pp. 225–230.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

