17-Beta Hydroxysteroid Dehydrogenase 13 Deficiency Does Not Protect Mice From Obesogenic Diet Injury
- PMID: 32779242
- PMCID: PMC8627256
- DOI: 10.1002/hep.31517
17-Beta Hydroxysteroid Dehydrogenase 13 Deficiency Does Not Protect Mice From Obesogenic Diet Injury
Abstract
Background and aims: 17-Beta hydroxysteroid dehydrogenase 13 (HSD17B13) is genetically associated with human nonalcoholic fatty liver disease (NAFLD). Inactivating mutations in HSD17B13 protect humans from NAFLD-associated and alcohol-associated liver injury, fibrosis, cirrhosis, and hepatocellular carcinoma, leading to clinical trials of anti-HSD17B13 therapeutic agents in humans. We aimed to study the in vivo function of HSD17B13 using a mouse model.
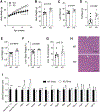
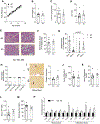
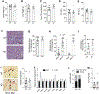
Approach and results: Single-cell RNA-sequencing and quantitative RT-PCR data revealed that hepatocytes are the main HSD17B13-expressing cells in mice and humans. We compared Hsd17b13 whole-body knockout (KO) mice and wild-type (WT) littermate controls fed regular chow (RC), a high-fat diet (HFD), a Western diet (WD), or the National Institute on Alcohol Abuse and Alcoholism model of alcohol exposure. HFD and WD induced significant weight gain, hepatic steatosis, and inflammation. However, there was no difference between genotypes with regard to body weight, liver weight, hepatic triglycerides (TG), histological inflammatory scores, expression of inflammation-related and fibrosis-related genes, and hepatic retinoid levels. Compared to WT, KO mice on the HFD had hepatic enrichment of most cholesterol esters, monoglycerides, and certain sphingolipid species. Extended feeding with the WD for 10 months led to extensive liver injury, fibrosis, and hepatocellular carcinoma, with no difference between genotypes. Under alcohol exposure, KO and WT mice showed similar hepatic TG and liver enzyme levels. Interestingly, chow-fed KO mice showed significantly higher body and liver weights compared to WT mice, while KO mice on obesogenic diets had a shift toward larger lipid droplets.
Conclusions: Extensive evaluation of Hsd17b13 deficiency in mice under several fatty liver-inducing dietary conditions did not reproduce the protective role of HSD17B13 loss-of-function mutants in human NAFLD. Moreover, mouse Hsd17b13 deficiency induces weight gain under RC. It is crucial to understand interspecies differences prior to leveraging HSD17B13 therapies.
© 2020 by the American Association for the Study of Liver Diseases.
Figures




Similar articles
-
Murine HSD17β13 does not control liver steatosis and modestly impacts fibrosis in a sex- and diet-specific manner.J Lipid Res. 2024 Oct;65(10):100634. doi: 10.1016/j.jlr.2024.100634. Epub 2024 Aug 24. J Lipid Res. 2024. PMID: 39182609 Free PMC article.
-
17-Beta Hydroxysteroid Dehydrogenase 13 Is a Hepatic Retinol Dehydrogenase Associated With Histological Features of Nonalcoholic Fatty Liver Disease.Hepatology. 2019 Apr;69(4):1504-1519. doi: 10.1002/hep.30350. Epub 2019 Mar 5. Hepatology. 2019. PMID: 30415504 Free PMC article.
-
Liver-specific Prkn knockout mice are more susceptible to diet-induced hepatic steatosis and insulin resistance.Mol Metab. 2020 Nov;41:101051. doi: 10.1016/j.molmet.2020.101051. Epub 2020 Jul 10. Mol Metab. 2020. PMID: 32653576 Free PMC article.
-
Role of HSD17B13 in the liver physiology and pathophysiology.Mol Cell Endocrinol. 2019 Jun 1;489:119-125. doi: 10.1016/j.mce.2018.10.014. Epub 2018 Oct 24. Mol Cell Endocrinol. 2019. PMID: 30365983 Review.
-
Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma.Int J Mol Sci. 2020 Feb 23;21(4):1525. doi: 10.3390/ijms21041525. Int J Mol Sci. 2020. PMID: 32102237 Free PMC article. Review.
Cited by
-
HSD17B13: A Potential Therapeutic Target for NAFLD.Front Mol Biosci. 2022 Jan 7;8:824776. doi: 10.3389/fmolb.2021.824776. eCollection 2021. Front Mol Biosci. 2022. PMID: 35071330 Free PMC article. Review.
-
HSD17B13 and other liver fat-modulating genes predict development of hepatocellular carcinoma among HCV-positive cirrhotics with and without viral clearance after DAA treatment.Clin J Gastroenterol. 2022 Apr;15(2):301-309. doi: 10.1007/s12328-021-01578-1. Epub 2022 Jan 31. Clin J Gastroenterol. 2022. PMID: 35098490
-
A 3D primary human cell-based in vitro model of non-alcoholic steatohepatitis for efficacy testing of clinical drug candidates.Sci Rep. 2021 Nov 23;11(1):22765. doi: 10.1038/s41598-021-01951-7. Sci Rep. 2021. PMID: 34815444 Free PMC article.
-
Advances in pediatric non-alcoholic fatty liver disease: From genetics to lipidomics.World J Clin Pediatr. 2022 Mar 23;11(3):221-238. doi: 10.5409/wjcp.v11.i3.221. eCollection 2022 May 9. World J Clin Pediatr. 2022. PMID: 35663007 Free PMC article. Review.
-
Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH).Signal Transduct Target Ther. 2022 Aug 13;7(1):287. doi: 10.1038/s41392-022-01119-3. Signal Transduct Target Ther. 2022. PMID: 35963848 Free PMC article. Review.
References
-
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. - PubMed
-
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

