TRPM8 Channel Activation Reduces the Spontaneous Contractions in Human Distal Colon
- PMID: 32751347
- PMCID: PMC7432081
- DOI: 10.3390/ijms21155403
TRPM8 Channel Activation Reduces the Spontaneous Contractions in Human Distal Colon
Abstract
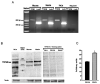
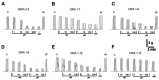
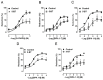
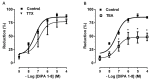
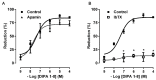
The transient receptor potential-melastatin 8 (TRPM8) is a non-selective Ca2+-permeable channel, activated by cold, membrane depolarization, and different cooling compounds. TRPM8 expression has been found in gut mucosal, submucosal, and muscular nerve endings. Although TRPM8 plays a role in pathological conditions, being involved in visceral pain and inflammation, the physiological functions in the digestive system remain unclear as yet. The aims of the present study were: (i) to verify the TRPM8 expression in human distal colon; (ii) to examine the effects of TRPM8 activation on colonic contractility; (iii) to characterize the mechanism of action. Reverse transcriptase-polymerase chain reaction (RT-PCR) and western blotting were used to analyze TRPM8 expression. The responses of human colon circular strips to different TRPM8 agonists [1-[Dialkyl-phosphinoyl]-alkane (DAPA) 2-5, 1-[Diisopropyl-phosphinoyl]-alkane (DIPA) 1-7, DIPA 1-8, DIPA 1-9, DIPA 1-10, and DIPA 1-12) were recorded using a vertical organ bath. The biomolecular analysis revealed gene and protein expression of TRPM8 in both mucosal and smooth muscle layers. All the agonists tested, except-DIPA 1-12, produced a concentration-dependent decrease in spontaneous contraction amplitude. The effect was significantly antagonized by 5-benzyloxytryptamine, a TRPM8 antagonist. The DIPA 1-8 agonist resulted in the most efficacious and potent activation among the tested molecules. The DIPA 1-8 effects were not affected by tetrodotoxin, a neural blocker, but they were significantly reduced by tetraethylammonium chloride, a non-selective blocker of K+ channels. Moreover, iberiotoxin, a blocker of the large-conductance Ca2+-dependent K+-channels, but not apamin, a blocker of small-conductance Ca2+-dependent K+ channels, significantly reduced the inhibitory DIPA 1-8 actions. The results of the present study demonstrated that TRPM8 receptors are also expressed in human distal colon in healthy conditions and that ligand-dependent TRPM8 activation is able to reduce the colonic spontaneous motility, probably by the opening of the large-conductance Ca2+-dependent K+-channels.
Keywords: 1-[Diisopropyl-phosphinoyl]-alkane (DIPA); IBS; TRPM-8; human colon contractility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effects of menthol on circular smooth muscle of human colon: analysis of the mechanism of action.Eur J Pharmacol. 2014 Oct 5;740:295-301. doi: 10.1016/j.ejphar.2014.07.018. Epub 2014 Jul 18. Eur J Pharmacol. 2014. PMID: 25046841
-
Transient receptor potential A1 is involved in cold-induced contraction in the isolated rat colon smooth muscle.Sheng Li Xue Bao. 2010 Aug 25;62(4):349-56. Sheng Li Xue Bao. 2010. PMID: 20717636
-
Inhibitory action of oxytocin on spontaneous contraction of rat distal colon by nitrergic mechanism: involvement of cyclic GMP and apamin-sensitive K+ channels.Acta Physiol (Oxf). 2017 Nov;221(3):182-192. doi: 10.1111/apha.12890. Epub 2017 May 20. Acta Physiol (Oxf). 2017. PMID: 28444988
-
The emerging pharmacology of TRPM8 channels: hidden therapeutic potential underneath a cold surface.Curr Pharm Biotechnol. 2011 Jan 1;12(1):54-67. doi: 10.2174/138920111793937916. Curr Pharm Biotechnol. 2011. PMID: 20932258 Review.
-
TRPM8 biology and medicinal chemistry.Curr Top Med Chem. 2011;11(17):2237-52. doi: 10.2174/156802611796904933. Curr Top Med Chem. 2011. PMID: 21671871 Review.
Cited by
-
The Role of Ion Channels in Functional Gastrointestinal Disorders (FGID): Evidence of Channelopathies and Potential Avenues for Future Research and Therapeutic Targets.Int J Mol Sci. 2023 Jul 4;24(13):11074. doi: 10.3390/ijms241311074. Int J Mol Sci. 2023. PMID: 37446251 Free PMC article. Review.
-
The Role and Function of TRPM8 in the Digestive System.Biomolecules. 2024 Jul 21;14(7):877. doi: 10.3390/biom14070877. Biomolecules. 2024. PMID: 39062591 Free PMC article. Review.
-
Effect of maternal curcumin supplementation on intestinal damage and the gut microbiota in male mice offspring with intra-uterine growth retardation.Eur J Nutr. 2022 Jun;61(4):1875-1892. doi: 10.1007/s00394-021-02783-x. Epub 2022 Jan 21. Eur J Nutr. 2022. PMID: 35059786
-
Advances in TRP channel drug discovery: from target validation to clinical studies.Nat Rev Drug Discov. 2022 Jan;21(1):41-59. doi: 10.1038/s41573-021-00268-4. Epub 2021 Sep 15. Nat Rev Drug Discov. 2022. PMID: 34526696 Free PMC article. Review.
-
Association Between the Cool Temperature-dependent Suppression of Colonic Peristalsis and Transient Receptor Potential Melastatin 8 Activation in Both a Randomized Clinical Trial and an Animal Model.J Neurogastroenterol Motil. 2022 Oct 30;28(4):693-705. doi: 10.5056/jnm21198. J Neurogastroenterol Motil. 2022. PMID: 36250375 Free PMC article.
References
-
- De Jong P.R., Takahashi N., Peiris M., Bertin S., Lee J., Gareau M.G., Paniagua A., Harris A.R., Herdman D.S., Corr M., et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunol. 2015;8:491–504. doi: 10.1038/mi.2014.82. - DOI - PMC - PubMed
-
- Almaraz L., Manenschijn J.A., de la Peña E., Viana F. TRPM8. In: Nilius B., Flockerzi V., editors. Mammalian Transient Receptor Potential (TRP) Cation Channels. Springer; Berlin/Heidelberg, Germany: 2014.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

