Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients
- PMID: 32707096
- PMCID: PMC7368656
- DOI: 10.1016/j.chom.2020.07.005
Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients
Abstract
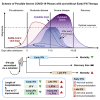
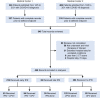
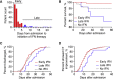
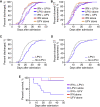
Interferons (IFNs) are widely used in treating coronavirus disease 2019 (COVID-19) patients. However, a recent report of ACE2, the host factor mediating SARS-Cov-2 infection, identifying it as interferon-stimulated raised considerable safety concern. To examine the association between the use and timing of IFN-α2b and clinical outcomes, we analyzed in a retrospective multicenter cohort study of 446 COVID-19 patients in Hubei, China. Regression models estimated that early administration (≤5 days after admission) of IFN-α2b was associated with reduced in-hospital mortality in comparison with no admission of IFN-α2b, whereas late administration of IFN-α2b was associated with increased mortality. Among survivors, early IFN-α2b was not associated with hospital discharge or computed tomography (CT) scan improvement, whereas late IFN-α2b was associated with delayed recovery. Additionally, early IFN-α2b and umifenovir alone or together were associated with reduced mortality and accelerated recovery in comparison with treatment with lopinavir/ritonavir (LPV/r) alone. We concluded that administration of IFN-α2b during the early stage of COVID-19 could induce favorable clinical responses.
Keywords: RNA virus; anti-retroviral agents; anti-viral immunity; cytokine storm syndrome; infectious disease; respiratory medicine; viral infection.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
Similar articles
-
Therapeutic Effectiveness of Interferon-α2b Against COVID-19: The Cuban Experience.J Interferon Cytokine Res. 2020 Sep;40(9):438-442. doi: 10.1089/jir.2020.0124. J Interferon Cytokine Res. 2020. PMID: 32960147
-
Quadruple therapy for asymptomatic COVID-19 infection patients.Expert Rev Anti Infect Ther. 2020 Jul;18(7):617-624. doi: 10.1080/14787210.2020.1758066. Epub 2020 May 3. Expert Rev Anti Infect Ther. 2020. PMID: 32362193 Free PMC article.
-
Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study.Microbes Infect. 2020 May-Jun;22(4-5):200-205. doi: 10.1016/j.micinf.2020.05.012. Epub 2020 May 20. Microbes Infect. 2020. PMID: 32445881 Free PMC article.
-
BET 1: Lopinavir-ritonavir and COVID-19.Emerg Med J. 2020 Jul;37(7):450-451. doi: 10.1136/emermed-2020-210221.2. Emerg Med J. 2020. PMID: 32616658 Review. No abstract available.
-
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00399-20. doi: 10.1128/AAC.00399-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152082 Free PMC article. Review.
Cited by
-
Future applications of host direct therapies for infectious disease treatment.Front Immunol. 2024 Oct 1;15:1436557. doi: 10.3389/fimmu.2024.1436557. eCollection 2024. Front Immunol. 2024. PMID: 39411713 Free PMC article. Review.
-
The interaction of innate immune and adaptive immune system.MedComm (2020). 2024 Sep 15;5(10):e714. doi: 10.1002/mco2.714. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39286776 Free PMC article. Review.
-
An intranasal nanoparticle STING agonist protects against respiratory viruses in animal models.Nat Commun. 2024 Jul 18;15(1):6053. doi: 10.1038/s41467-024-50234-y. Nat Commun. 2024. PMID: 39025863 Free PMC article.
-
Innate Immunity in Protection and Pathogenesis During Coronavirus Infections and COVID-19.Annu Rev Immunol. 2024 Jun;42(1):615-645. doi: 10.1146/annurev-immunol-083122-043545. Annu Rev Immunol. 2024. PMID: 38941608 Review.
-
Beneficial and Detrimental Effects of Cytokines during Influenza and COVID-19.Viruses. 2024 Feb 18;16(2):308. doi: 10.3390/v16020308. Viruses. 2024. PMID: 38400083 Free PMC article. Review.
References
-
- National Health Commission of the People’s Republic of China; 2020. Chinese management guideline for COVID-19 (version 7)http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb19... [in Chinese] - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

