Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen
- PMID: 32661364
- PMCID: PMC7442749
- DOI: 10.1038/s41590-020-0725-2
Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen
Abstract
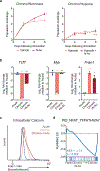
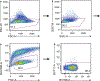
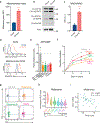
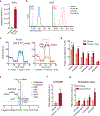
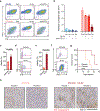
The majority of tumor-infiltrating T cells exhibit a terminally exhausted phenotype, marked by a loss of self-renewal capacity. How repetitive antigenic stimulation impairs T cell self-renewal remains poorly defined. Here, we show that persistent antigenic stimulation impaired ADP-coupled oxidative phosphorylation. The resultant bioenergetic compromise blocked proliferation by limiting nucleotide triphosphate synthesis. Inhibition of mitochondrial oxidative phosphorylation in activated T cells was sufficient to suppress proliferation and upregulate genes linked to T cell exhaustion. Conversely, prevention of mitochondrial oxidative stress during chronic T cell stimulation allowed sustained T cell proliferation and induced genes associated with stem-like progenitor T cells. As a result, antioxidant treatment enhanced the anti-tumor efficacy of chronically stimulated T cells. These data reveal that loss of ATP production through oxidative phosphorylation limits T cell proliferation and effector function during chronic antigenic stimulation. Furthermore, treatments that maintain redox balance promote T cell self-renewal and enhance anti-tumor immunity.
Figures





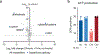





Comment in
-
Mitochondrial Damage and the Road to Exhaustion.Cell Metab. 2020 Dec 1;32(6):905-907. doi: 10.1016/j.cmet.2020.11.004. Cell Metab. 2020. PMID: 33264601
Similar articles
-
Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion.Nat Immunol. 2021 Feb;22(2):205-215. doi: 10.1038/s41590-020-00834-9. Epub 2021 Jan 4. Nat Immunol. 2021. PMID: 33398183 Free PMC article.
-
Impaired mitochondrial oxidative phosphorylation induces CD8+ T cell exhaustion.Biochem Biophys Res Commun. 2024 Nov 19;734:150738. doi: 10.1016/j.bbrc.2024.150738. Epub 2024 Sep 24. Biochem Biophys Res Commun. 2024. PMID: 39342799
-
Activation of NKT Cells in an Anti-PD-1-Resistant Tumor Model Enhances Antitumor Immunity by Reinvigorating Exhausted CD8 T Cells.Cancer Res. 2018 Sep 15;78(18):5315-5326. doi: 10.1158/0008-5472.CAN-18-0734. Epub 2018 Jul 16. Cancer Res. 2018. PMID: 30012672
-
Signaling defects in anti-tumor T cells.Immunol Rev. 2008 Apr;222:192-205. doi: 10.1111/j.1600-065X.2008.00606.x. Immunol Rev. 2008. PMID: 18364003 Free PMC article. Review.
-
Effector, Memory, and Dysfunctional CD8(+) T Cell Fates in the Antitumor Immune Response.J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. Epub 2016 May 22. J Immunol Res. 2016. PMID: 27314056 Free PMC article. Review.
Cited by
-
Methylmalonic acid induces metabolic abnormalities and exhaustion in CD8+ T cells to suppress anti-tumor immunity.Oncogene. 2025 Jan;44(2):105-114. doi: 10.1038/s41388-024-03191-1. Epub 2024 Oct 29. Oncogene. 2025. PMID: 39472497
-
Promises and challenges of adoptive T-cell therapies for solid tumours.Br J Cancer. 2021 May;124(11):1759-1776. doi: 10.1038/s41416-021-01353-6. Epub 2021 Mar 29. Br J Cancer. 2021. PMID: 33782566 Free PMC article. Review.
-
Enhanced T cell immune activity mediated by Drp1 promotes the efficacy of PD-1 inhibitors in treating lung cancer.Cancer Immunol Immunother. 2024 Feb 10;73(2):40. doi: 10.1007/s00262-023-03582-5. Cancer Immunol Immunother. 2024. PMID: 38340166 Free PMC article.
-
Reprogramming T-cell metabolism to enhance adoptive cell therapies.Int Immunol. 2024 Apr 27;36(6):261-278. doi: 10.1093/intimm/dxae007. Int Immunol. 2024. PMID: 38364321 Review.
-
A glance through the effects of CD4+ T cells, CD8+ T cells, and cytokines on Alzheimer's disease.Comput Struct Biotechnol J. 2023 Nov 8;21:5662-5675. doi: 10.1016/j.csbj.2023.10.058. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 38053545 Free PMC article. Review.
References
-
- Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). - PubMed
-
- Siddiqui I et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 50, 195–211 e110 (2019). - PubMed
Methods-only references
-
- Anders S et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nature protocols 8, 1765–1786 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

