Quantification of the CM-Dil-labeled human umbilical cord mesenchymal stem cells migrated to the dual injured uterus in SD rat
- PMID: 32660551
- PMCID: PMC7359016
- DOI: 10.1186/s13287-020-01806-4
Quantification of the CM-Dil-labeled human umbilical cord mesenchymal stem cells migrated to the dual injured uterus in SD rat
Abstract
Background: Human umbilical cord mesenchymal stem cell (hUC-MSC) therapy is considered as a promising approach in the treatment of intrauterine adhesions (IUAs). Considerable researches have already detected hUC-MSCs by diverse methods. This paper aims at exploring the quantitative distribution of CM-Dil-labeled hUC-MSCs in different regions of the uterus tissue of the dual injury-induced IUAs in rats and the underlying mechanism of restoration of fertility after implantation of hUC-MSCs in the IUA model.
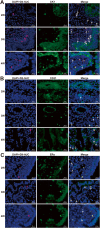
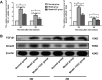
Methods: In this study, we investigated the quantification of the CM-Dil-labeled hUC-MSCs migrated to the dual injured uterus in Sprague Dawley rats. Additionally, we investigated the differentiation of CM-Dil-labeled hUC-MSCs. The differentiation potential of epithelial cells, vascular endothelial cells, and estrogen receptor (ER) cells were assessed by an immunofluorescence method using CK7, CD31, and ERα. The therapeutic impact of hUC-MSCs in the IUA model was assessed by hematoxylin and eosin, Masson, immunohistochemistry staining, and reproductive function test. Finally, the expression of TGF-β1/Smad3 pathway in uterine tissues was determined by qRT-PCR and Western blotting.
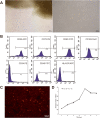
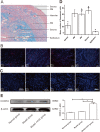
Results: The CM-Dil-labeled cells in the stroma region were significantly higher than those in the superficial myometrium (SM) (71.67 ± 7.98 vs. 60.92 ± 3.96, p = 0.005), in the seroma (71.67 ± 7.98 vs. 23.67 ± 8.08, p = 0.000) and in the epithelium (71.67 ± 7.98 vs. 4.17 ± 1.19, p = 0.000). From the 2nd week of treatment, hUC-MSCs began to differentiate into epithelial cells, vascular endothelial cells, and ER cells. The therapeutic group treated with hUC-MSCs exhibited a significant decrease in fibrosis (TGF-β1/Smad3) as well as a significant increase in vascularization (CD31) compared with the untreated rats.
Conclusion: Our findings suggested that the distribution of the migrated hUC-MSCs in different regions of the uterine tissue was unequal. Most cells were in the stroma and less were in the epithelium of endometrium and gland. Injected hUC-MSCs had a capacity to differentiate into epithelial cells, vascular endothelial cells, and ER cells; increase blood supply; inhibit fibration; and then restore the fertility of the IUA model.
Keywords: CM-Dil; Differentiate; Human umbilical cord mesenchymal stem cells; Intrauterine adhesions; Quantification.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Human umbilical cord-derived mesenchymal stem cells and auto-crosslinked hyaluronic acid gel complex for treatment of intrauterine adhesion.Aging (Albany NY). 2024 Apr 1;16(7):6273-6289. doi: 10.18632/aging.205704. Epub 2024 Apr 1. Aging (Albany NY). 2024. PMID: 38568100 Free PMC article.
-
Human Umbilical Cord Mesenchymal Stem Cells Promote Anti-Inflammation and Angiogenesis by Targeting Macrophages in a Rat Uterine Scar Model.Stem Cell Rev Rep. 2024 Aug;20(6):1555-1568. doi: 10.1007/s12015-024-10730-6. Epub 2024 May 4. Stem Cell Rev Rep. 2024. PMID: 38703310
-
Human umbilical cord blood-derived MSCs trans-differentiate into endometrial cells and regulate Th17/Treg balance through NF-κB signaling in rabbit intrauterine adhesions endometrium.Stem Cell Res Ther. 2022 Jul 15;13(1):301. doi: 10.1186/s13287-022-02990-1. Stem Cell Res Ther. 2022. PMID: 35841027 Free PMC article.
-
What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment?Stem Cell Res Ther. 2020 Dec 1;11(1):519. doi: 10.1186/s13287-020-02011-z. Stem Cell Res Ther. 2020. PMID: 33261658 Free PMC article. Review.
-
Human Umbilical Cord Mesenchymal Stem Cells' Cultivation and Treatment of Liver Diseases.Curr Stem Cell Res Ther. 2023;18(3):286-298. doi: 10.2174/1574888X17666220623111406. Curr Stem Cell Res Ther. 2023. PMID: 35747963 Review.
Cited by
-
Research progress of stem cell therapy for endometrial injury.Mater Today Bio. 2022 Aug 8;16:100389. doi: 10.1016/j.mtbio.2022.100389. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36033375 Free PMC article. Review.
-
Bi-potential hPSC-derived Müllerian duct-like cells for full-thickness and functional endometrium regeneration.NPJ Regen Med. 2022 Nov 23;7(1):68. doi: 10.1038/s41536-022-00263-2. NPJ Regen Med. 2022. PMID: 36418304 Free PMC article.
-
Human Umbilical Cord-Based Therapeutics: Stem Cells and Blood Derivatives for Female Reproductive Medicine.Int J Mol Sci. 2022 Dec 14;23(24):15942. doi: 10.3390/ijms232415942. Int J Mol Sci. 2022. PMID: 36555583 Free PMC article. Review.
-
Injectable conductive gelatin methacrylate / oxidized dextran hydrogel encapsulating umbilical cord mesenchymal stem cells for myocardial infarction treatment.Bioact Mater. 2021 Nov 17;13:119-134. doi: 10.1016/j.bioactmat.2021.11.011. eCollection 2022 Jul. Bioact Mater. 2021. PMID: 35224296 Free PMC article.
-
17β-Estradiol promotes angiogenesis of bone marrow mesenchymal stem cells by upregulating the PI3K-Akt signaling pathway.Comput Struct Biotechnol J. 2022 Jul 19;20:3864-3873. doi: 10.1016/j.csbj.2022.07.028. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35891776 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

