Echinatin suppresses esophageal cancer tumor growth and invasion through inducing AKT/mTOR-dependent autophagy and apoptosis
- PMID: 32655130
- PMCID: PMC7354992
- DOI: 10.1038/s41419-020-2730-7
Echinatin suppresses esophageal cancer tumor growth and invasion through inducing AKT/mTOR-dependent autophagy and apoptosis
Abstract
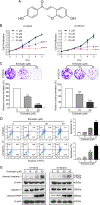
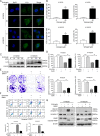
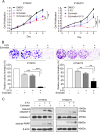
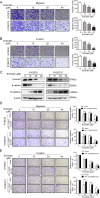
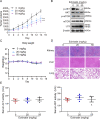
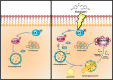
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors with poor survival. It is urgent to search for new efficient drugs with good stability and safety for clinical therapy. This study aims to identify potential anticancer drugs from a compound library consisting of 429 natural products. Echinatin, a compound isolated from the Chinese herb Glycyrrhiza uralensis Fisch, was found to markedly induce apoptosis and inhibit proliferation and colony-formation ability in ESCC. Confocal fluorescence microscopy data showed that echinatin significantly induced autophagy in ESCC cells, and autophagy inhibitor bafilomycinA1 attenuated the suppressive effects of echinatin on cell viability and apoptosis. Mechanistically, RNA sequencing coupled with bioinformatics analysis and a series of functional assays revealed that echinatin induced apoptosis and autophagy through inactivation of AKT/mTOR signaling pathway, whereas constitutive activation of AKT significantly abrogated these effects. Furthermore, we demonstrated that echinatin had a significant antitumor effect in the tumor xenograft model and markedly suppressed cell migration and invasion abilities of ESCC cells in a dose-dependent manner. Our findings provide the first evidence that echinatin could be a novel therapeutic strategy for treating ESCC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Downregulation of fibulin-4 inhibits autophagy and promotes the sensitivity of esophageal squamous cell carcinoma cells to apatinib by activating the Akt-mTOR signaling pathway.Thorac Cancer. 2022 Sep;13(18):2592-2605. doi: 10.1111/1759-7714.14595. Epub 2022 Aug 11. Thorac Cancer. 2022. PMID: 35950373 Free PMC article.
-
DEPTOR suppresses the progression of esophageal squamous cell carcinoma and predicts poor prognosis.Oncotarget. 2016 Mar 22;7(12):14188-98. doi: 10.18632/oncotarget.7420. Oncotarget. 2016. PMID: 26893358 Free PMC article.
-
The ginsenoside Rk3 exerts anti-esophageal cancer activity in vitro and in vivo by mediating apoptosis and autophagy through regulation of the PI3K/Akt/mTOR pathway.PLoS One. 2019 May 15;14(5):e0216759. doi: 10.1371/journal.pone.0216759. eCollection 2019. PLoS One. 2019. PMID: 31091245 Free PMC article.
-
Natural products targeting autophagy via the PI3K/Akt/mTOR pathway as anticancer agents.Anticancer Agents Med Chem. 2013 Sep;13(7):1048-56. doi: 10.2174/18715206113139990130. Anticancer Agents Med Chem. 2013. PMID: 23293890 Review.
-
Cryptotanshinone inhibits oral squamous cell carcinoma through the autophagic pathway.Neoplasma. 2023 Feb;70(1):114-122. doi: 10.4149/neo_2023_220924N957. Epub 2023 Jan 27. Neoplasma. 2023. PMID: 36704921 Review.
Cited by
-
Maprotiline Suppresses Cholesterol Biosynthesis and Hepatocellular Carcinoma Progression Through Direct Targeting of CRABP1.Front Pharmacol. 2021 May 20;12:689767. doi: 10.3389/fphar.2021.689767. eCollection 2021. Front Pharmacol. 2021. PMID: 34093212 Free PMC article.
-
Phytochemicals Mediate Autophagy Against Osteoarthritis by Maintaining Cartilage Homeostasis.Front Pharmacol. 2021 Dec 20;12:795058. doi: 10.3389/fphar.2021.795058. eCollection 2021. Front Pharmacol. 2021. PMID: 34987406 Free PMC article. Review.
-
Echinatin mitigates sevoflurane-induced neurotoxicity through regulation of ferroptosis and iron homeostasis.Aging (Albany NY). 2024 Mar 5;16(5):4670-4683. doi: 10.18632/aging.205622. Epub 2024 Mar 5. Aging (Albany NY). 2024. PMID: 38446592 Free PMC article.
-
Blockade of Nuclear β-Catenin Signaling via Direct Targeting of RanBP3 with NU2058 Induces Cell Senescence to Suppress Colorectal Tumorigenesis.Adv Sci (Weinh). 2022 Dec;9(34):e2202528. doi: 10.1002/advs.202202528. Epub 2022 Oct 21. Adv Sci (Weinh). 2022. PMID: 36270974 Free PMC article.
-
Application of Natural Medicinal Plants Active Ingredients in Oral Squamous Cell Carcinoma.Chin J Integr Med. 2024 Sep;30(9):852-864. doi: 10.1007/s11655-024-3804-7. Epub 2024 Apr 12. Chin J Integr Med. 2024. PMID: 38607612 Review.
References
-
- Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2018;68:394–424. - PubMed
-
- Xie X, et al. Gossypetin is a novel MKK3 and MKK6 inhibitor that suppresses esophageal cancer growth in vitro and in vivo. Cancer Lett. 2019;442:126–136. - PubMed
-
- Wang Y, et al. Liensinine perchlorate inhibits colorectal cancer tumorigenesis by inducing mitochondrial dysfunction and apoptosis. Food Funct. 2018;9:5536–5546. - PubMed
-
- Takeuchi H, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann. Surg. 2014;260:259–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

