Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques
- PMID: 32636454
- PMCID: PMC7364749
- DOI: 10.1038/s41422-020-0364-z
Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques
Abstract
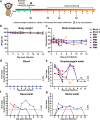
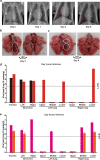
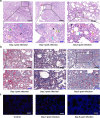
The 2019 novel coronavirus (SARS-CoV-2) outbreak is a major challenge for public health. SARS-CoV-2 infection in human has a broad clinical spectrum ranging from mild to severe cases, with a mortality rate of ~6.4% worldwide (based on World Health Organization daily situation report). However, the dynamics of viral infection, replication and shedding are poorly understood. Here, we show that Rhesus macaques are susceptible to the infection by SARS-CoV-2. After intratracheal inoculation, the first peak of viral RNA was observed in oropharyngeal swabs one day post infection (1 d.p.i.), mainly from the input of the inoculation, while the second peak occurred at 5 d.p.i., which reflected on-site replication in the respiratory tract. Histopathological observation shows that SARS-CoV-2 infection can cause interstitial pneumonia in animals, characterized by hyperemia and edema, and infiltration of monocytes and lymphocytes in alveoli. We also identified SARS-CoV-2 RNA in respiratory tract tissues, including trachea, bronchus and lung; and viruses were also re-isolated from oropharyngeal swabs, bronchus and lung, respectively. Furthermore, we demonstrated that neutralizing antibodies generated from the primary infection could protect the Rhesus macaques from a second-round challenge by SARS-CoV-2. The non-human primate model that we established here provides a valuable platform to study SARS-CoV-2 pathogenesis and to evaluate candidate vaccines and therapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques.Nat Commun. 2020 Sep 2;11(1):4400. doi: 10.1038/s41467-020-18149-6. Nat Commun. 2020. PMID: 32879306 Free PMC article.
-
Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques.Science. 2020 Aug 14;369(6505):818-823. doi: 10.1126/science.abc5343. Epub 2020 Jul 2. Science. 2020. PMID: 32616673 Free PMC article.
-
SARS-CoV-2 infection protects against rechallenge in rhesus macaques.Science. 2020 Aug 14;369(6505):812-817. doi: 10.1126/science.abc4776. Epub 2020 May 20. Science. 2020. PMID: 32434946 Free PMC article.
-
Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2.Nature. 2020 Sep;585(7824):273-276. doi: 10.1038/s41586-020-2423-5. Epub 2020 Jun 9. Nature. 2020. PMID: 32516797 Free PMC article.
-
The 2020 Pandemic: Current SARS-CoV-2 Vaccine Development.Front Immunol. 2020 Aug 19;11:1880. doi: 10.3389/fimmu.2020.01880. eCollection 2020. Front Immunol. 2020. PMID: 32973779 Free PMC article. Review.
Cited by
-
Advocacy and Activism as Essential Tools in Primate Conservation.Int J Primatol. 2022;43(1):168-184. doi: 10.1007/s10764-021-00201-x. Epub 2021 Mar 10. Int J Primatol. 2022. PMID: 33716363 Free PMC article.
-
The human-primate interface in the New Normal: Challenges and opportunities for primatologists in the COVID-19 era and beyond.Am J Primatol. 2020 Aug;82(8):e23176. doi: 10.1002/ajp.23176. Epub 2020 Jul 20. Am J Primatol. 2020. PMID: 32686188 Free PMC article.
-
SARS CoV-2 infections in animals, two years into the pandemic.Arch Virol. 2022 Dec;167(12):2503-2517. doi: 10.1007/s00705-022-05609-1. Epub 2022 Oct 7. Arch Virol. 2022. PMID: 36207554 Free PMC article. Review.
-
Immunopathogenesis of SARS-CoV-2-induced pneumonia: lessons from influenza virus infection.Inflamm Regen. 2020 Oct 12;40:39. doi: 10.1186/s41232-020-00148-1. eCollection 2020. Inflamm Regen. 2020. PMID: 33062077 Free PMC article. Review.
-
A betacoronavirus multiplex microsphere immunoassay detects early SARS-CoV-2 seroconversion and controls for pre-existing seasonal human coronavirus antibody cross-reactivity.medRxiv [Preprint]. 2020 Oct 16:2020.10.14.20207050. doi: 10.1101/2020.10.14.20207050. medRxiv. 2020. PMID: 33083807 Free PMC article. Preprint.
References
-
- WHO. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) (2020).
-
- WHO. Coronavirus disease 2019 (COVID-19) Situation Report—119 (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

