Half a century of amyloids: past, present and future
- PMID: 32632432
- PMCID: PMC7445747
- DOI: 10.1039/c9cs00199a
Half a century of amyloids: past, present and future
Abstract
Amyloid diseases are global epidemics with profound health, social and economic implications and yet remain without a cure. This dire situation calls for research into the origin and pathological manifestations of amyloidosis to stimulate continued development of new therapeutics. In basic science and engineering, the cross-β architecture has been a constant thread underlying the structural characteristics of pathological and functional amyloids, and realizing that amyloid structures can be both pathological and functional in nature has fuelled innovations in artificial amyloids, whose use today ranges from water purification to 3D printing. At the conclusion of a half century since Eanes and Glenner's seminal study of amyloids in humans, this review commemorates the occasion by documenting the major milestones in amyloid research to date, from the perspectives of structural biology, biophysics, medicine, microbiology, engineering and nanotechnology. We also discuss new challenges and opportunities to drive this interdisciplinary field moving forward.
Conflict of interest statement
Conflicts of interest
DSE is SAB chair and equity holder in ADDRx, Inc.
Figures


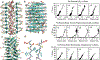





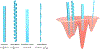

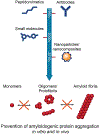

Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Dynamic Field Theory of Executive Function: Identifying Early Neurocognitive Markers.Monogr Soc Res Child Dev. 2024 Dec;89(3):7-109. doi: 10.1111/mono.12478. Monogr Soc Res Child Dev. 2024. PMID: 39628288 Free PMC article.
-
Place-Based Developmental Research: Conceptual and Methodological Advances in Studying Youth Development in Context.Monogr Soc Res Child Dev. 2023 Dec;88(3):7-130. doi: 10.1111/mono.12472. Monogr Soc Res Child Dev. 2023. PMID: 37953661 Free PMC article.
-
Australia in 2030: what is our path to health for all?Med J Aust. 2021 May;214 Suppl 8:S5-S40. doi: 10.5694/mja2.51020. Med J Aust. 2021. PMID: 33934362
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
The membrane axis of Alzheimer's nanomedicine.Adv Nanobiomed Res. 2021 Jan;1(1):2000040. doi: 10.1002/anbr.202000040. Epub 2020 Nov 26. Adv Nanobiomed Res. 2021. PMID: 33748816 Free PMC article.
-
Massive experimental quantification of amyloid nucleation allows interpretable deep learning of protein aggregation.bioRxiv [Preprint]. 2024 Oct 1:2024.07.13.603366. doi: 10.1101/2024.07.13.603366. bioRxiv. 2024. PMID: 39071305 Free PMC article. Preprint.
-
Proteomic Evidence for Amyloidogenic Cross-Seeding in Fibrinaloid Microclots.Int J Mol Sci. 2024 Oct 8;25(19):10809. doi: 10.3390/ijms251910809. Int J Mol Sci. 2024. PMID: 39409138 Free PMC article.
-
Effect of Cross-Seeding of Wild-Type Amyloid-β1-40 Peptides with Post-translationally Modified Fibrils on Internal Dynamics of the Fibrils Using Deuterium Solid-State NMR.J Phys Chem B. 2023 Apr 6;127(13):2887-2899. doi: 10.1021/acs.jpcb.2c07817. Epub 2023 Mar 23. J Phys Chem B. 2023. PMID: 36952330 Free PMC article.
-
Protein nanofibrils and their use as building blocks of sustainable materials.RSC Adv. 2021 Dec 8;11(62):39188-39215. doi: 10.1039/d1ra06878d. eCollection 2021 Dec 6. RSC Adv. 2021. PMID: 35492452 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

