Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health
- PMID: 32625968
- PMCID: PMC7009542
- DOI: 10.2903/j.efsa.2018.5327
Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health
Abstract
The European Food Safety Authority has produced this Guidance on human and animal health aspects (Part 1) of the risk assessment of nanoscience and nanotechnology applications in the food and feed chain. It covers the application areas within EFSA's remit, e.g. novel foods, food contact materials, food/feed additives and pesticides. The Guidance takes account of the new developments that have taken place since publication of the previous Guidance in 2011. Potential future developments are suggested in the scientific literature for nanoencapsulated delivery systems and nanocomposites in applications such as novel foods, food/feed additives, biocides, pesticides and food contact materials. Therefore, the Guidance has taken account of relevant new scientific studies that provide more insights to physicochemical properties, exposure assessment and hazard characterisation of nanomaterials. It specifically elaborates on physicochemical characterisation of nanomaterials in terms of how to establish whether a material is a nanomaterial, the key parameters that should be measured, the methods and techniques that can be used for characterisation of nanomaterials and their determination in complex matrices. It also details the aspects relating to exposure assessment and hazard identification and characterisation. In particular, nanospecific considerations relating to in vivo/in vitro toxicological studies are discussed and a tiered framework for toxicological testing is outlined. It describes in vitro degradation, toxicokinetics, genotoxicity as well as general issues relating to testing of nanomaterials. Depending on the initial tier results, studies may be needed to investigate reproductive and developmental toxicity, immunotoxicity, allergenicity, neurotoxicity, effects on gut microbiome and endocrine activity. The possible use of read-across to fill data gaps as well as the potential use of integrated testing strategies and the knowledge of modes/mechanisms of action are also discussed. The Guidance proposes approaches to risk characterisation and uncertainty analysis, and provides recommendations for further research in this area.
Keywords: Nanomaterial; feed; food; guidance; nanoscience; nanotechnology; risk assessment; testing strategy.
© 2018 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
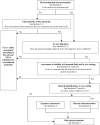
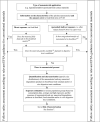
(a): If it cannot be measured whether a nanomaterial is present in e.g. food/feed matrix, food simulant or simulated digestive tract, it should be assumed it is. See Section 6.2.1 and Appendix D for details on in vitro gastrointestinal digestion.
(b): The assessment of degradation products that are still in the form of a nanomaterial should continue as presented in this Guidance.
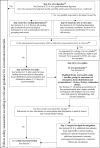
(a): If yes, an argument can be put forward that further nanospecific testing is not necessary. However, it is anticipated that for most cases that have entered Step 1, testing under Step 2 will be required. This is because conclusive evidence for toxicity (including local effects for any relevant route of exposure) based on in vitro testing alone, is not expected. Hence, the decision to not perform any toxicity study has also implication for uncertainty reporting (see Section 8.3). Direct testing under Step 2 is acceptable to demonstrate if the nanomaterial represents a hazard or not. Furthermore, for many Regulatory frameworks, e.g. for food additives, there is a requirement for a 90‐day test. In these cases, this study has to be designed according to the stipulations of nanospecific issues as described in this Guidance for performing the tests of Steps 2 and 3.
(b): Review of existing information should continue throughout the entire process of hazard identification and risk assessment.
(c): Step 1 genotoxcity testing is mandatory for all nanomaterial. A positive result in Step 1 requires follow‐up in Step 2.
(d): Some nanomaterials have been related to inflammation, immunotoxicity, genotoxicity, reproductive organ effects and/or neurotoxicity (Dekkers et al., ; Bencsik et al., ; Higashisaka et al., ; Prosafe white paper, 2017). Indications for respective effects during step 1 and 2 assessment should be further investigated in Step 3.
Similar articles
-
Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health.EFSA J. 2021 Aug 3;19(8):e06768. doi: 10.2903/j.efsa.2021.6768. eCollection 2021 Aug. EFSA J. 2021. PMID: 34377190 Free PMC article.
-
Eating nanomaterials: cruelty-free and safe? the EFSA guidance on risk assessment of nanomaterials in food and feed.Altern Lab Anim. 2011 Dec;39(6):567-75. doi: 10.1177/026119291103900611. Altern Lab Anim. 2011. PMID: 22338723
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles.EFSA J. 2021 Aug 3;19(8):e06769. doi: 10.2903/j.efsa.2021.6769. eCollection 2021 Aug. EFSA J. 2021. PMID: 34377191 Free PMC article.
-
EFSA's OpenFoodTox: An open source toxicological database on chemicals in food and feed and its future developments.Environ Int. 2021 Jan;146:106293. doi: 10.1016/j.envint.2020.106293. Epub 2020 Dec 8. Environ Int. 2021. PMID: 33395940 Review.
Cited by
-
Physical, chemical, and toxicological characterization of fibrillated forms of cellulose using an in vitro gastrointestinal digestion and co-culture model.Toxicol Res (Camb). 2020 May 20;9(3):290-301. doi: 10.1093/toxres/tfaa026. eCollection 2020 Jun. Toxicol Res (Camb). 2020. PMID: 32670560 Free PMC article.
-
The Physicochemical Properties of Graphene Nanocomposites Influence the Anticancer Effect.J Oncol. 2019 Jul 3;2019:7254534. doi: 10.1155/2019/7254534. eCollection 2019. J Oncol. 2019. PMID: 31354821 Free PMC article. Review.
-
Biomolecules from Macroalgae-Nutritional Profile and Bioactives for Novel Food Product Development.Biomolecules. 2023 Feb 17;13(2):386. doi: 10.3390/biom13020386. Biomolecules. 2023. PMID: 36830755 Free PMC article. Review.
-
NCs-Delivered Pesticides: A Promising Candidate in Smart Agriculture.Int J Mol Sci. 2021 Dec 2;22(23):13043. doi: 10.3390/ijms222313043. Int J Mol Sci. 2021. PMID: 34884846 Free PMC article. Review.
-
Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health.EFSA J. 2021 Aug 3;19(8):e06768. doi: 10.2903/j.efsa.2021.6768. eCollection 2021 Aug. EFSA J. 2021. PMID: 34377190 Free PMC article.
References
-
- Ammendolia MG, Iosi F, Maranghi F, Tassinari R, Cubadda F, Aureli F, Raggi A, Superti F, Mantovani A and De Berardis B, 2017. Short‐term oral exposure to low doses of nano‐sized TiO2 and potential modulatory effects on intestinal cells. Food and Chemical Toxicology, 102, 63–75. 10.1016/j.fct.2017.01.031 - DOI - PubMed
-
- Arts JHE, Hadi M, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG, Warheit D, Wiench K and Landsiedel R, 2014. A critical appraisal of existing concepts for the grouping of nanomaterials. Regulatory Toxicology and Pharmacology, 70, 492–506. 10.1016/j.yrtph.2014.07.025 - DOI - PubMed
-
- Arts JHE, Hadi M, Irfan M‐A, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG, Warheit D, Wiench K, Wohlleben W and Landsiedel R, 2015. A decision‐making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regulatory Toxicology and Pharmacology, 71(2 Suppl), S1–S27. 10.1016/j.yrtph.2015.03.007 - DOI - PubMed
-
- Arts JHE, Irfan M‐A, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Neubauer N, Petry T, Sauer UG, Warheit D, Wiench K, Wohlleben W and Landsiedel R, 2016. Case studies putting the decision‐making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regulatory Toxicology and Pharmacology, 76, 234261. 10.1016/j.yrtph.2015.11.020 - DOI - PubMed
LinkOut - more resources
Full Text Sources
