Can neoadjuvant chemotherapy improve survival in stage T3-4N1 nasopharyngeal carcinoma? A propensity matched analysis
- PMID: 32615984
- PMCID: PMC7331182
- DOI: 10.1186/s13014-020-01594-4
Can neoadjuvant chemotherapy improve survival in stage T3-4N1 nasopharyngeal carcinoma? A propensity matched analysis
Abstract
Background: To estimate the efficacy of neoadjuvant chemotherapy (NCT) in stage T3-4N1 nasopharyngeal carcinoma (NPC).
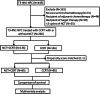
Methods: Data on stage T3-4N1 NPC patients treated with concurrent chemoradiotherapy (CCRT) with or without NCT at the Sun Yat-sen University Cancer Center between January 2006 and December 2013 were retrospectively reviewed. Propensity score matching (PSM) was carried out to balance prognostic factors in NCT followed by CCRT (NCT + CCRT) group and CCRT group in a 1:1 ratio. Survival outcomes of matched patients in the two groups were compared, and prognostic factors were identified using Cox regression model.
Results: A total of 282 patients were involved in this study, with 136 of NCT + CCRT group and 146 of CCRT group. After PSM, 85 pairs of patients were selected. There were no significant differences in 5-year overall survival (OS), locoregional recurrence-free survival (LRFS), distant recurrence-free survival (DRFS), and recurrence-free survival (RFS) between NCT + CCRT group and CCRT group (81.0% vs. 77.5%, P = 0.750; 85.8% vs. 88.1%, P = 0.495; 92.5% vs. 93.9%, P = 0.759; 81.0% vs.77.5%, P = 0.919, respectively). Multivariate analysis found that smoking history (P = 0.044) and T classification (P = 0.027) were independent prognostic factors for OS, lymph node diameter (P = 0.032) was independent prognostic factor for LRFS, positive pretreatment lymph node condition (PLNC), which was defined as the lymph node necrosis or confluent, was independent prognostic factor for DRFS (P = 0.007), and RFS (P = 0.009). Lower 5-year OS (82.7% vs. 94.1%, P = 0.014), DRFS (79.3% vs. 96.2%, P = 0.003), and RFS (62.4% vs. 86.8%, P = 0.001) were found in positive PLNC group compared with negative PLNC group. In terms of toxicities, the incidences of acute hematological Grade 3-4 adverse events (AEs) were higher in NCT + CCRT group compared with CCRT group (P < 0.05), while no significant difference was observed in the rates of non-hematological Grade 3-4 AEs between these two groups (P > 0.05).
Conclusions: Additional NCT is not associated with improved survival outcomes for patients with stage T3-4N1 NPC, but bring increased hematological Grade 3-4 AEs. PLNC is independent prognostic factor in stage T3-4N1 NPC, with positive PLNC correlating with poor survival outcomes.
Keywords: Intensity-modulated radiotherapy; Nasopharyngeal neoplasm; Neoadjuvant chemotherapy; Prognosis.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures

Similar articles
-
Neoadjuvant chemotherapy plus intensity-modulated radiotherapy versus neoadjuvant chemotherapy plus concurrent chemoradiotherapy for ascending or descending types of nasopharyngeal carcinoma: A retrospective study.Am J Otolaryngol. 2022 Jan-Feb;43(1):103193. doi: 10.1016/j.amjoto.2021.103193. Epub 2021 Sep 3. Am J Otolaryngol. 2022. PMID: 34509080
-
The efficacy of induction chemotherapy or adjuvant chemotherapy added to concurrent chemoradiotherapy in T3-4N0-1M0 nasopharyngeal carcinoma: a propensity score-matched analysis.Cancer Biol Ther. 2023 Dec 31;24(1):2274121. doi: 10.1080/15384047.2023.2274121. Epub 2023 Nov 15. Cancer Biol Ther. 2023. PMID: 37965924 Free PMC article.
-
The Role of Neoadjuvant Chemotherapy in the Treatment of Nasopharyngeal Carcinoma: A Multi-institutional Retrospective Study (KROG 11-06) Using Propensity Score Matching Analysis.Cancer Res Treat. 2016 Jul;48(3):917-27. doi: 10.4143/crt.2015.265. Epub 2015 Dec 28. Cancer Res Treat. 2016. PMID: 26727716 Free PMC article.
-
Concurrent chemoradiotherapy with additional chemotherapy for nasopharyngeal carcinoma: A pooled analysis of propensity score-matching studies.Head Neck. 2021 Jun;43(6):1912-1927. doi: 10.1002/hed.26664. Epub 2021 Feb 28. Head Neck. 2021. PMID: 33644916 Review.
-
Role of chemotherapy in stage IIb nasopharyngeal carcinoma.Chin J Cancer. 2012 Dec;31(12):573-8. doi: 10.5732/cjc.011.10433. Epub 2012 Jul 4. Chin J Cancer. 2012. PMID: 22776232 Free PMC article. Review.
Cited by
-
A Nomogram to Identify the Optimal Candidates for Induction Chemotherapy in Advanced N-Stage Nasopharyngeal Carcinoma.Cancer Manag Res. 2022 Aug 31;14:2583-2596. doi: 10.2147/CMAR.S377731. eCollection 2022. Cancer Manag Res. 2022. PMID: 36068822 Free PMC article.
References
-
- Lee AW, Lau WH, Tung SY, Chua DT, Chappell R, Xu L, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong nasopharyngeal Cancer study group. J Clin Oncol. 2005;23:6966–6975. doi: 10.1200/JCO.2004.00.7542. - DOI - PubMed
-
- Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi: 10.1016/j.radonc.2012.08.013. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

