PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine?
- PMID: 32605290
- PMCID: PMC7397204
- DOI: 10.3390/genes11070719
PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine?
Abstract
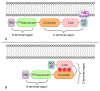
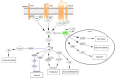
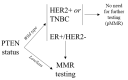
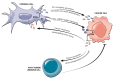
Alterations in the tumor suppressor phosphatase and tensin homolog (PTEN) occur in a substantial proportion of solid tumors. These events drive tumorigenesis and tumor progression. Given its central role as a downregulator of the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway, PTEN is deeply involved in cell growth, proliferation, and survival. This gene is also implicated in the modulation of the DNA damage response and in tumor immune microenvironment modeling. Despite the actionability of PTEN alterations, their role as biomarkers remains controversial in clinical practice. To date, there is still a substantial lack of validated guidelines and/or recommendations for PTEN testing. Here, we provide an update on the current state of knowledge on biologic and genetic alterations of PTEN across the most frequent solid tumors, as well as on their actual and/or possible clinical applications. We focus on possible tailored schemes for cancer patients' clinical management, including risk assessment, diagnosis, prognostication, and treatment.
Keywords: PI3K/Akt; PTEN; biomarker; cancer; precision medicine; solid tumors; tumor immune microenvironment; tumor suppressor.
Conflict of interest statement
N.F. has received honoraria for consulting/advisory role from Merck Sharp & Dohme (MSD) and Boehringer Ingelheim. E.G.R. has received honoraria for advisory role/speaker bureau from Thermo Fisher Scientific, Roche, Novartis, AstraZeneca. C.Cr. has received honoraria for consulting/advisory role/speaker bureau from Novartis, Eli-Lilly, Pfizer, Roche. U.M. reports personal fees from Boehringer Ingelheim, AstraZeneca, Roche, MSD, Amgen, Merck, and BMS for participation in a speaker bureau or for acting in an advisory role, outside the submitted work. M.I. has received consultation honoraria from Errekappa Euroterapici S.p.a. These companies had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, and/or in the decision to publish the results. All the other authors declare no conflicts of interest.
Figures

Similar articles
-
Frequent PTEN loss and differential HER2/PI3K signaling pathway alterations in salivary duct carcinoma: Implications for targeted therapy.Cancer. 2018 Sep 15;124(18):3693-3705. doi: 10.1002/cncr.31600. Epub 2018 Oct 5. Cancer. 2018. PMID: 30289966 Free PMC article.
-
MicroRNA-146b promotes PI3K/AKT pathway hyperactivation and thyroid cancer progression by targeting PTEN.Oncogene. 2018 Jun;37(25):3369-3383. doi: 10.1038/s41388-017-0088-9. Epub 2018 Jan 22. Oncogene. 2018. PMID: 29353884
-
Emerging role of PTEN loss in evasion of the immune response to tumours.Br J Cancer. 2020 Jun;122(12):1732-1743. doi: 10.1038/s41416-020-0834-6. Epub 2020 Apr 24. Br J Cancer. 2020. PMID: 32327707 Free PMC article. Review.
-
The PTEN Conundrum: How to Target PTEN-Deficient Prostate Cancer.Cells. 2020 Oct 22;9(11):2342. doi: 10.3390/cells9112342. Cells. 2020. PMID: 33105713 Free PMC article. Review.
-
Pathway-based identification of biomarkers for targeted therapeutics: personalized oncology with PI3K pathway inhibitors.Sci Transl Med. 2010 Aug 4;2(43):43ra55. doi: 10.1126/scitranslmed.3001065. Sci Transl Med. 2010. PMID: 20686178
Cited by
-
HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes.Life (Basel). 2022 Sep 9;12(9):1403. doi: 10.3390/life12091403. Life (Basel). 2022. PMID: 36143438 Free PMC article. Review.
-
Mismatch repair-deficient hormone receptor-positive breast cancers: Biology and pathological characterization.Cancer Cell Int. 2021 May 17;21(1):266. doi: 10.1186/s12935-021-01976-y. Cancer Cell Int. 2021. PMID: 34001143 Free PMC article. Review.
-
S6K1 Controls DNA Damage Signaling Modulated by the MRN Complex to Induce Radioresistance in Lung Cancer.Int J Mol Sci. 2024 Sep 28;25(19):10461. doi: 10.3390/ijms251910461. Int J Mol Sci. 2024. PMID: 39408794 Free PMC article.
-
A novel mutation in PTEN in anaplastic thyroid carcinoma: A case report.Biomed Rep. 2024 Jul 2;21(2):127. doi: 10.3892/br.2024.1815. eCollection 2024 Aug. Biomed Rep. 2024. PMID: 39006510 Free PMC article.
-
The Genetic Architecture of Vascular Anomalies: Current Data and Future Therapeutic Perspectives Correlated with Molecular Mechanisms.Int J Mol Sci. 2022 Oct 13;23(20):12199. doi: 10.3390/ijms232012199. Int J Mol Sci. 2022. PMID: 36293054 Free PMC article. Review.
References
-
- Steck P.A., Pershouse M.A., Jasser S.A., Yung W.K., Lin H., Ligon A.H., Langford L.A., Baumgard M.L., Hattier T., Davis T., et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

