Polymeric Composite Dressings Containing Calcium-Releasing Nanoparticles Accelerate Wound Healing in Diabetic Mice
- PMID: 32602814
- PMCID: PMC8082736
- DOI: 10.1089/wound.2020.1206
Polymeric Composite Dressings Containing Calcium-Releasing Nanoparticles Accelerate Wound Healing in Diabetic Mice
Abstract
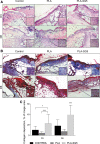
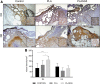
Objective: Wound healing is a complex process that involves the interaction between different cell types and bioactive factors. Impaired wound healing is characterized by a loss in synchronization of these interactions, resulting in nonhealing chronic wounds. Chronic wounds are a socioeconomic burden, one of the most prominent clinical manifestations of diabetes, however, they lack satisfactory treatment options. The objective of this study was to develop polymeric composites that deliver ions having wound healing properties and evaluate its performance using a pressure ulcer model in diabetic mice. Approach: To develop a polymeric composite wound dressing containing ion-releasing nanoparticles for chronic wound healing. This composite was chemically and physically characterized and evaluated using a pressure ulcer wound model in diabetic (db/db) mice to explore their potential as novel wound dressing. Results: This dressing exhibits a controlled ion release and a good in vitro bioactivity. The polymeric composite dressing treatment stimulates angiogenesis, collagen synthesis, granulation tissue formation, and accelerates wound closure of ischemic wounds created in diabetic mice. In addition, the performance of the newly designed composite is remarkably better than a commercially available dressing frequently used for the treatment of low-exuding chronic wounds. Innovation: The developed nanoplatforms are cell- and growth factor free and control the host microenvironment resulting in enhanced wound healing. These nanoplatforms are available by cost-effective synthesis with a defined composition, offering an additional advantage in potential clinical application. Conclusion: Based on the obtained results, these polymeric composites offer an optimum approach for chronic wound healing without adding cells or external biological factors.
Keywords: angiogenesis; bioactive dressings; chronic wounds; diabetes.
Conflict of interest statement
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
Figures


Similar articles
-
Oxygen Generating Polymeric Nano Fibers That Stimulate Angiogenesis and Show Efficient Wound Healing in a Diabetic Wound Model.Int J Nanomedicine. 2020 May 18;15:3511-3522. doi: 10.2147/IJN.S248911. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32547010 Free PMC article.
-
Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release.ACS Nano. 2019 Sep 24;13(9):10279-10293. doi: 10.1021/acsnano.9b03656. Epub 2019 Sep 9. ACS Nano. 2019. PMID: 31483606
-
Functionalized PVA-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling.J Tissue Eng Regen Med. 2018 Mar;12(3):e1559-e1570. doi: 10.1002/term.2581. Epub 2017 Nov 5. J Tissue Eng Regen Med. 2018. PMID: 28987032
-
Recent Advances in Polymer-based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-art.Curr Drug Targets. 2018;19(5):527-550. doi: 10.2174/1389450118666170704132523. Curr Drug Targets. 2018. PMID: 28676002 Review.
-
Antibacterial biohybrid nanofibers for wound dressings.Acta Biomater. 2020 Apr 15;107:25-49. doi: 10.1016/j.actbio.2020.02.022. Epub 2020 Feb 19. Acta Biomater. 2020. PMID: 32084600 Review.
Cited by
-
Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements.Cells. 2022 Aug 6;11(15):2439. doi: 10.3390/cells11152439. Cells. 2022. PMID: 35954282 Free PMC article. Review.
-
Ag-Contained Superabsorbent Curdlan-Chitosan Foams for Healing Wounds in a Type-2 Diabetic Mice Model.Pharmaceutics. 2022 Mar 28;14(4):724. doi: 10.3390/pharmaceutics14040724. Pharmaceutics. 2022. PMID: 35456559 Free PMC article.
-
Polysaccharide-based hydrogel enriched by epidermal growth factor peptide fragment for improving the wound healing process.Heliyon. 2023 Nov 23;9(12):e22749. doi: 10.1016/j.heliyon.2023.e22749. eCollection 2023 Dec. Heliyon. 2023. PMID: 38094045 Free PMC article.
-
Advance in topical biomaterials and mechanisms for the intervention of pressure injury.iScience. 2023 May 26;26(6):106956. doi: 10.1016/j.isci.2023.106956. eCollection 2023 Jun 16. iScience. 2023. PMID: 37378311 Free PMC article. Review.
-
The Role of Calcium in Wound Healing.Int J Mol Sci. 2021 Jun 17;22(12):6486. doi: 10.3390/ijms22126486. Int J Mol Sci. 2021. PMID: 34204292 Free PMC article. Review.
References
-
- Guariguata L, Whiting DR, Hambleton I, Beagley J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2013;103:137–149 - PubMed
-
- Castaño O, Pérez-Amodio S, Navarro C, Mateos-timoneda Á, Engel E. Instructive microenvironments in skin wound healing: biomaterials as signal releasing platforms. Adv Drug Deliv Rev 2018;129:95–117 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

