TDP-43 Is Efficiently Transferred Between Neuron-Like Cells in a Manner Enhanced by Preservation of Its N-Terminus but Independent of Extracellular Vesicles
- PMID: 32595443
- PMCID: PMC7301158
- DOI: 10.3389/fnins.2020.00540
TDP-43 Is Efficiently Transferred Between Neuron-Like Cells in a Manner Enhanced by Preservation of Its N-Terminus but Independent of Extracellular Vesicles
Abstract
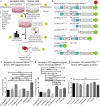
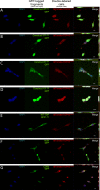
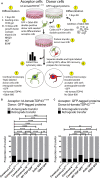
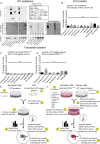
The misfolding of transactive response DNA-binding protein (TDP-43) is a major contributor to the pathogenesis of TDP-43 proteinopathies, including amyotrophic lateral sclerosis and frontotemporal lobar degeneration with TDP-43 inclusions, but also plays a role in other neurodegenerative diseases including Alzheimer disease. It is thought that different truncations at the N- and C-termini of TDP-43 contribute to its misfolding and aggregation in the brain, and that these aberrant TDP-43 fragments contribute to disease. Despite this, little is known about whether different truncation events influence the protein's transmissibility between cells and how this cell-to-cell transfer occurs. In this study, we use a well-established cellular model to study the efficiency by which full-length and truncated TDP-43 fragments are transferred between neuron-like cells. We demonstrate that preservation of the N-terminus of TDP-43 enhances its transmissibility between cells and that this protein transmission occurs in a manner exclusive of extracellular vesicles, instead requiring cellular proximity for efficient propagation. These data indicate that the N-terminus of TDP-43 might be a useful target in the generation of therapeutics to limit the spread of TDP-43 pathology.
Keywords: C-terminus; N-terminus; TDP-43; amyotrophic lateral sclerosis; cell-to-cell; extracellular vesicles; frontotemporal lobar degeneration; protein transfer.
Copyright © 2020 Sackmann, Sackmann and Hallbeck.
Figures
Similar articles
-
The role of transactive response DNA-binding protein-43 in amyotrophic lateral sclerosis and frontotemporal dementia.Curr Opin Neurol. 2008 Dec;21(6):693-700. doi: 10.1097/WCO.0b013e3283168d1d. Curr Opin Neurol. 2008. PMID: 18989115 Free PMC article. Review.
-
TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis.Neurosignals. 2008;16(1):41-51. doi: 10.1159/000109758. Epub 2007 Dec 5. Neurosignals. 2008. PMID: 18097159 Review.
-
Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments.Brain. 2011 Sep;134(Pt 9):2610-26. doi: 10.1093/brain/awr159. Epub 2011 Jul 13. Brain. 2011. PMID: 21752789
-
TDP-43 cytoplasmic inclusion formation is disrupted in C9orf72-associated amyotrophic lateral sclerosis/frontotemporal lobar degeneration.Brain Commun. 2019;1(1):fcz014. doi: 10.1093/braincomms/fcz014. Epub 2019 Sep 11. Brain Commun. 2019. PMID: 31633109 Free PMC article.
-
Fluorescence in-situ hybridization method reveals that carboxyl-terminal fragments of transactive response DNA-binding protein-43 truncated at the amino acid residue 218 reduce poly(A)+ RNA expression.Neuroreport. 2018 Jul 4;29(10):846-851. doi: 10.1097/WNR.0000000000001042. Neuroreport. 2018. PMID: 29742622 Free PMC article.
Cited by
-
Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis.Lancet Neurol. 2022 May;21(5):465-479. doi: 10.1016/S1474-4422(21)00414-2. Epub 2022 Mar 22. Lancet Neurol. 2022. PMID: 35334234 Free PMC article. Review.
-
Mechanisms of TDP-43 Proteinopathy Onset and Propagation.Int J Mol Sci. 2021 Jun 2;22(11):6004. doi: 10.3390/ijms22116004. Int J Mol Sci. 2021. PMID: 34199367 Free PMC article. Review.
-
Amyotrophic Lateral Sclerosis: Proteins, Proteostasis, Prions, and Promises.Front Cell Neurosci. 2020 Nov 4;14:581907. doi: 10.3389/fncel.2020.581907. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33328890 Free PMC article.
-
Amyotrophic lateral sclerosis.Lancet. 2022 Oct 15;400(10360):1363-1380. doi: 10.1016/S0140-6736(22)01272-7. Epub 2022 Sep 15. Lancet. 2022. PMID: 36116464 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

