Auxiliary role of mesenchymal stem cells as regenerative medicine soldiers to attenuate inflammatory processes of severe acute respiratory infections caused by COVID-19
- PMID: 32588163
- PMCID: PMC7315014
- DOI: 10.1007/s10561-020-09842-3
Auxiliary role of mesenchymal stem cells as regenerative medicine soldiers to attenuate inflammatory processes of severe acute respiratory infections caused by COVID-19
Abstract
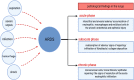
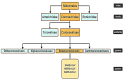
Acute respiratory infections as one of the most common problems of healthcare systems also can be considered as an important reason for worldwide morbidity and mortality from infectious diseases. Coronaviruses are a group of well-known respiratory viruses that can cause acute respiratory infections. At the current state, the 2019 novel coronavirus is cited as the most worldwide problematic agent for the respiratory system. According to investigations, people with old age and underlying diseases are at higher risk of 2019 novel coronavirus infection. Indeed, they may show a severe form of the disease (with severe acute respiratory infections). Based on the promising role of cell therapy and regenerative medicine approaches in the treatment of several life-threatening diseases, it seems that applying cell-based approaches can also be a hopeful strategy for improving subjects with severe acute respiratory infections caused by the 2019 novel coronavirus. Herein, due to the amazing effects of mesenchymal stem cells in the treatment of various diseases, this review focuses on the auxiliary role of mesenchymal stem cells to reduce inflammatory processes of acute respiratory infections caused by the 2019 novel coronavirus.
Keywords: ARDS; COVID-19; Cell therapy; Coronavirus; Mesenchymal stem cells; Pneumonia; Regenerative medicine.
Conflict of interest statement
There is no conflict of interest.
Figures


Similar articles
-
Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics.Protein Cell. 2020 Oct;11(10):707-722. doi: 10.1007/s13238-020-00738-2. Epub 2020 Jun 9. Protein Cell. 2020. PMID: 32519302 Free PMC article. Review.
-
Immunomodulation and Regeneration Properties of Dental Pulp Stem Cells: A Potential Therapy to Treat Coronavirus Disease 2019.Cell Transplant. 2020 Jan-Dec;29:963689720952089. doi: 10.1177/0963689720952089. Cell Transplant. 2020. PMID: 32830527 Free PMC article. Review.
-
Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use.Cells. 2020 Apr 9;9(4):924. doi: 10.3390/cells9040924. Cells. 2020. PMID: 32283815 Free PMC article.
-
Mesenchymal stem cells: current clinical progress in ARDS and COVID-19.Stem Cell Res Ther. 2020 Jul 22;11(1):305. doi: 10.1186/s13287-020-01804-6. Stem Cell Res Ther. 2020. PMID: 32698898 Free PMC article. Review.
-
Mesenchymal stromal cells and their secreted extracellular vesicles as therapeutic tools for COVID-19 pneumonia?J Control Release. 2020 Sep 10;325:135-140. doi: 10.1016/j.jconrel.2020.06.036. Epub 2020 Jul 3. J Control Release. 2020. PMID: 32622963 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cell-Derived Exosomes in Various Chronic Liver Diseases: Hype or Hope?J Inflamm Res. 2024 Jan 10;17:171-189. doi: 10.2147/JIR.S439974. eCollection 2024. J Inflamm Res. 2024. PMID: 38223423 Free PMC article. Review.
-
Nursing diagnoses in hospitalized patients with COVID-19 in Indonesia.Belitung Nurs J. 2022 Feb 22;8(1):44-52. doi: 10.33546/bnj.1828. eCollection 2022. Belitung Nurs J. 2022. PMID: 37521083 Free PMC article.
-
Critical roles of cytokine storm and bacterial infection in patients with COVID-19: therapeutic potential of mesenchymal stem cells.Inflammopharmacology. 2023 Feb;31(1):171-206. doi: 10.1007/s10787-022-01132-6. Epub 2023 Jan 4. Inflammopharmacology. 2023. PMID: 36600055 Free PMC article. Review.
-
Progress in COVID research and developments during pandemic.View (Beijing). 2022 Jul 20:20210020. doi: 10.1002/VIW.20210020. Online ahead of print. View (Beijing). 2022. PMID: 35941909 Free PMC article. Review.
-
COVID-19 Pathology on Various Organs and Regenerative Medicine and Stem Cell-Based Interventions.Front Cell Dev Biol. 2021 Jun 14;9:675310. doi: 10.3389/fcell.2021.675310. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34195193 Free PMC article. Review.
References
-
- Aghayan HR, Goodarzi P, Arjmand B (2014) GMP-compliant human adipose tissue-derived mesenchymal stem cells for cellular therapy. In: Stem cells and good manufacturing practices, Springer, New York, pp 93–107 - PubMed
-
- Arjmand B, Larijani B, Heshmat R, Norouzi-Javidan A, Soleimani M, Aghayan HR. The current state of clinical cell transplantation trials in Iran: a survey in 2011. Arch NeuroSci. 2014;1:7–14.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

