A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2
- PMID: 32571838
- PMCID: PMC7319273
- DOI: 10.1126/science.abc6952
A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2
Abstract
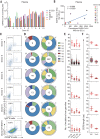
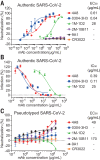
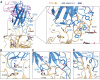
Developing therapeutics against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could be guided by the distribution of epitopes, not only on the receptor binding domain (RBD) of the Spike (S) protein but also across the full Spike (S) protein. We isolated and characterized monoclonal antibodies (mAbs) from 10 convalescent COVID-19 patients. Three mAbs showed neutralizing activities against authentic SARS-CoV-2. One mAb, named 4A8, exhibits high neutralization potency against both authentic and pseudotyped SARS-CoV-2 but does not bind the RBD. We defined the epitope of 4A8 as the N-terminal domain (NTD) of the S protein by determining with cryo-eletron microscopy its structure in complex with the S protein to an overall resolution of 3.1 angstroms and local resolution of 3.3 angstroms for the 4A8-NTD interface. This points to the NTD as a promising target for therapeutic mAbs against COVID-19.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures



Similar articles
-
Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody.Nature. 2020 Jul;583(7815):290-295. doi: 10.1038/s41586-020-2349-y. Epub 2020 May 18. Nature. 2020. PMID: 32422645
-
Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike.Nature. 2020 Aug;584(7821):450-456. doi: 10.1038/s41586-020-2571-7. Epub 2020 Jul 22. Nature. 2020. PMID: 32698192
-
Targeting SARS-CoV2 Spike Protein Receptor Binding Domain by Therapeutic Antibodies.Biomed Pharmacother. 2020 Oct;130:110559. doi: 10.1016/j.biopha.2020.110559. Epub 2020 Aug 1. Biomed Pharmacother. 2020. PMID: 32768882 Free PMC article. Review.
-
Analysis of a SARS-CoV-2-Infected Individual Reveals Development of Potent Neutralizing Antibodies with Limited Somatic Mutation.Immunity. 2020 Jul 14;53(1):98-105.e5. doi: 10.1016/j.immuni.2020.06.001. Epub 2020 Jun 8. Immunity. 2020. PMID: 32561270 Free PMC article.
-
Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2.Signal Transduct Target Ther. 2020 Sep 22;5(1):212. doi: 10.1038/s41392-020-00318-0. Signal Transduct Target Ther. 2020. PMID: 32963228 Free PMC article. Review.
Cited by
-
Characterisation of B.1.1.7 and Pangolin coronavirus spike provides insights on the evolutionary trajectory of SARS-CoV-2.bioRxiv [Preprint]. 2021 Mar 22:2021.03.22.436468. doi: 10.1101/2021.03.22.436468. bioRxiv. 2021. PMID: 33791702 Free PMC article. Preprint.
-
Computational epitope map of SARS-CoV-2 spike protein.PLoS Comput Biol. 2021 Apr 1;17(4):e1008790. doi: 10.1371/journal.pcbi.1008790. eCollection 2021 Apr. PLoS Comput Biol. 2021. PMID: 33793546 Free PMC article.
-
Dynamics of B-cell repertoires and emergence of cross-reactive responses in COVID-19 patients with different disease severity.medRxiv [Preprint]. 2021 Apr 5:2020.07.13.20153114. doi: 10.1101/2020.07.13.20153114. medRxiv. 2021. Update in: Cell Rep. 2021 May 25;35(8):109173. doi: 10.1016/j.celrep.2021.109173. PMID: 32699862 Free PMC article. Updated. Preprint.
-
A Therapeutic Non-self-reactive SARS-CoV-2 Antibody Protects from Lung Pathology in a COVID-19 Hamster Model.Cell. 2020 Nov 12;183(4):1058-1069.e19. doi: 10.1016/j.cell.2020.09.049. Epub 2020 Sep 23. Cell. 2020. PMID: 33058755 Free PMC article.
-
Substance Use Disorder in the COVID-19 Pandemic: A Systematic Review of Vulnerabilities and Complications.Pharmaceuticals (Basel). 2020 Jul 18;13(7):155. doi: 10.3390/ph13070155. Pharmaceuticals (Basel). 2020. PMID: 32708495 Free PMC article. Review.
References
-
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. F., Tan W.; China Novel Coronavirus Investigating and Research Team , A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020). 10.1056/NEJMoa2001017 - DOI - PMC - PubMed
-
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E. C., Zhang Y.-Z., A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020). 10.1038/s41586-020-2008-3 - DOI - PMC - PubMed
-
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). 10.1016/S0140-6736(20)30183-5 - DOI - PMC - PubMed
-
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). 10.1038/s41586-020-2012-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

