Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial
- PMID: 32568367
- PMCID: PMC7309571
- DOI: 10.1001/jamaneurol.2020.1840
Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial
Abstract
Importance: Insulin modulates aspects of brain function relevant to Alzheimer disease and can be delivered to the brain using intranasal devices. To date, the use of intranasal insulin to treat persons with mild cognitive impairment and Alzheimer's disease dementia remains to be examined in a multi-site trial.
Objective: To examine the feasibility, safety, and efficacy of intranasal insulin for the treatment of persons with mild cognitive impairment and Alzheimer disease dementia in a phase 2/3 multisite clinical trial.
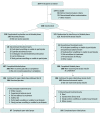
Design, setting, and participants: A randomized (1:1) double-blind clinical trial was conducted between 2014 and 2018. Participants received 40 IU of insulin or placebo for 12 months during the blinded phase, which was followed by a 6-month open-label extension phase. The clinical trial was conducted at 27 sites of the Alzheimer's Therapeutic Research Institute. A total of 432 adults were screened, and 144 adults were excluded. Inclusion criteria included adults aged 55 to 85 years with a diagnosis of amnestic mild cognitive impairment or Alzheimer disease (based on National Institute on Aging-Alzheimer Association criteria), a score of 20 or higher on the Mini-Mental State Examination, a clinical dementia rating of 0.5 or 1.0, and a delayed logical memory score within a specified range. A total of 289 participants were randomized. Among the first 49 participants, the first device (device 1) used to administer intranasal insulin treatment had inconsistent reliability. A new device (device 2) was used for the remaining 240 participants, who were designated the primary intention-to-treat population. Data were analyzed from August 2018 to March 2019.
Interventions: Participants received 40 IU of insulin (Humulin-RU-100; Lilly) or placebo (diluent) daily for 12 months (blinded phase) followed by a 6-month open-label extension phase. Insulin was administered with 2 intranasal delivery devices.
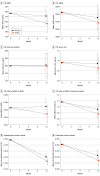
Main outcomes and measures: The primary outcome (mean score change on the Alzheimer Disease Assessment Scale-cognitive subscale 12) was evaluated at 3-month intervals. Secondary clinical outcomes were assessed at 6-month intervals. Cerebrospinal fluid collection and magnetic resonance imaging scans occurred at baseline and 12 months.
Results: A total of 289 participants (155 men [54.6%]; mean [SD] age, 70.9 [7.1] years) were randomized. Of those, 260 participants completed the blinded phase, and 240 participants completed the open-label extension phase. For the first 49 participants, the first device used to administer treatment had inconsistent reliability. A second device was used for the remaining 240 participants (123 men [51.3%]; mean [SD] age, 70.8 [7.1] years), who were designated the primary intention-to-treat population. No differences were observed between treatment arms for the primary outcome (mean score change on ADAS-cog-12 from baseline to month 12) in the device 2 ITT cohort (0.0258 points; 95% CI, -1.771 to 1.822 points; P = .98) or for the other clinical or cerebrospinal fluid outcomes in the primary (second device) intention-to-treat analysis. No clinically important adverse events were associated with treatment.
Conclusions and relevance: In this study, no cognitive or functional benefits were observed with intranasal insulin treatment over a 12-month period among the primary intention-to-treat cohort.
Trial registration: ClinicalTrials.gov Identifier: NCT01767909.
Conflict of interest statement
Figures


Similar articles
-
Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial.Arch Neurol. 2012 Jan;69(1):29-38. doi: 10.1001/archneurol.2011.233. Epub 2011 Sep 12. Arch Neurol. 2012. PMID: 21911655 Free PMC article. Clinical Trial.
-
Intranasal Insulin Reduces White Matter Hyperintensity Progression in Association with Improvements in Cognition and CSF Biomarker Profiles in Mild Cognitive Impairment and Alzheimer's Disease.J Prev Alzheimers Dis. 2021;8(3):240-248. doi: 10.14283/jpad.2021.14. J Prev Alzheimers Dis. 2021. PMID: 34101779 Free PMC article. Clinical Trial.
-
A Phase II, Single-Center, Randomized, Double-Blind, Placebo-Controlled Study of the Safety and Therapeutic Efficacy of Intranasal Glulisine in Amnestic Mild Cognitive Impairment and Probable Mild Alzheimer's Disease.Drugs Aging. 2021 May;38(5):407-415. doi: 10.1007/s40266-021-00845-7. Epub 2021 Mar 15. Drugs Aging. 2021. PMID: 33719017 Clinical Trial.
-
Metal protein attenuating compounds for the treatment of Alzheimer's dementia.Cochrane Database Syst Rev. 2014 Feb 21;2014(2):CD005380. doi: 10.1002/14651858.CD005380.pub5. Cochrane Database Syst Rev. 2014. PMID: 24563468 Free PMC article. Review.
-
Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 14-05198-EF-1. PMID: 24354019 Free Books & Documents. Review.
Cited by
-
Therapeutic Advances in Diabetes, Autoimmune, and Neurological Diseases.Int J Mol Sci. 2021 Mar 10;22(6):2805. doi: 10.3390/ijms22062805. Int J Mol Sci. 2021. PMID: 33802091 Free PMC article. Review.
-
Advanced Glycation End Products-Induced Alzheimer's Disease and Its Novel Therapeutic Approaches: A Comprehensive Review.Cureus. 2024 May 30;16(5):e61373. doi: 10.7759/cureus.61373. eCollection 2024 May. Cureus. 2024. PMID: 38947632 Free PMC article. Review.
-
A case for seeking sex-specific treatments in Alzheimer's disease.Front Aging Neurosci. 2024 Feb 13;16:1346621. doi: 10.3389/fnagi.2024.1346621. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38414633 Free PMC article. Review.
-
Insulin-inspired hippocampal neuron-targeting technology for protein drug delivery.Proc Natl Acad Sci U S A. 2024 Oct 8;121(41):e2407936121. doi: 10.1073/pnas.2407936121. Epub 2024 Sep 30. Proc Natl Acad Sci U S A. 2024. PMID: 39348543
-
Targeting whole body metabolism and mitochondrial bioenergetics in the drug development for Alzheimer's disease.Acta Pharm Sin B. 2022 Feb;12(2):511-531. doi: 10.1016/j.apsb.2021.06.014. Epub 2021 Jun 30. Acta Pharm Sin B. 2022. PMID: 35256932 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

