Effectiveness and safety of glucocorticoids to treat COVID-19: a rapid review and meta-analysis
- PMID: 32566564
- PMCID: PMC7290628
- DOI: 10.21037/atm-20-3307
Effectiveness and safety of glucocorticoids to treat COVID-19: a rapid review and meta-analysis
Abstract
Background: Glucocorticoids are widely used in the treatment of various pulmonary inflammatory diseases, but they are also often accompanied by significant adverse reactions. Published guidelines point out that low dose and short duration systemic glucocorticoid therapy may be considered for patients with rapidly progressing coronavirus disease 2019 (COVID-19) while the evidence is still limited.
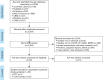
Methods: We comprehensively searched electronic databases and supplemented the screening by conducting a manual search. We included randomized controlled trials (RCTs) and cohort studies evaluating the effectiveness and safety of glucocorticoids in children and adults with COVID-19, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), and conducted meta-analyses of the main indicators that were identified in the studies.
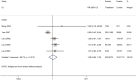
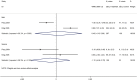
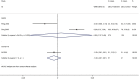
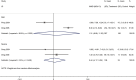
Results: Our search retrieved 23 studies, including one RCT and 22 cohort studies, with a total of 13,815 patients. In adults with COVID-19, the use of systemic glucocorticoid did not reduce mortality [risk ratio (RR) =2.00, 95% confidence interval (CI): 0.69 to 5.75, I2=90.9%] or the duration of lung inflammation [weighted mean difference (WMD) =-1 days, 95% CI: -2.91 to 0.91], while a significant reduction was found in the duration of fever (WMD =-3.23 days, 95% CI: -3.56 to -2.90). In patients with SARS, glucocorticoids also did not reduce the mortality (RR =1.52, 95% CI: 0.89 to 2.60, I2=84.6%), duration of fever (WMD =0.82 days, 95% CI: -2.88 to 4.52, I2=97.9%) or duration of lung inflammation absorption (WMD =0.95 days, 95% CI: -7.57 to 9.48, I2=94.6%). The use of systemic glucocorticoid therapy prolonged the duration of hospital stay in all patients (COVID-19, SARS and MERS).
Conclusions: Glucocorticoid therapy was found to reduce the duration of fever, but not mortality, duration of hospitalization or lung inflammation absorption. Long-term use of high-dose glucocorticoids increased the risk of adverse reactions such as coinfections, so routine use of systemic glucocorticoids for patients with COVID-19 cannot be recommend.
Keywords: Coronavirus disease 2019 (COVID-19); glucocorticoids; meta-analysis; rapid review.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3307). MSL serves as the unpaid editorial board member of Annals of Translational Medicine from Nov 2019 to Oct 2021. The other authors have no conflicts of interest to declare.
Figures







Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Jul 10;7(7):CD013600. doi: 10.1002/14651858.CD013600.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. PMID: 32648959 Free PMC article. Updated.
-
Convalescent plasma for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2023 May 10;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub6. Cochrane Database Syst Rev. 2023. PMID: 37162745 Free PMC article. Review.
-
Convalescent plasma for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2023 Feb 1;2(2):CD013600. doi: 10.1002/14651858.CD013600.pub5. Cochrane Database Syst Rev. 2023. Update in: Cochrane Database Syst Rev. 2023 May 10;5:CD013600. doi: 10.1002/14651858.CD013600.pub6. PMID: 36734509 Free PMC article. Updated. Review.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 20;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Feb 1;2:CD013600. doi: 10.1002/14651858.CD013600.pub5. PMID: 34013969 Free PMC article. Updated.
Cited by
-
Review of COVID-19 Treatment.J Res Pharm Pract. 2021 Jan 11;9(4):169-174. doi: 10.4103/jrpp.JRPP_20_72. eCollection 2020 Oct-Dec. J Res Pharm Pract. 2021. PMID: 33912498 Free PMC article. Review.
-
Evaluating the Risk-Benefit Profile of Corticosteroid Therapy for COVID-19 Patients: A Scoping Review.Pharmacy (Basel). 2024 Aug 22;12(4):129. doi: 10.3390/pharmacy12040129. Pharmacy (Basel). 2024. PMID: 39195858 Free PMC article.
-
Chemotherapy vs. Immunotherapy in combating nCOVID19: An update.Hum Immunol. 2021 Sep;82(9):649-658. doi: 10.1016/j.humimm.2021.05.001. Epub 2021 May 18. Hum Immunol. 2021. PMID: 34020832 Free PMC article. Review.
-
COVID-19 of differing severity: from bulk to single-cell expression data analysis.Cell Cycle. 2023 Jul-Aug;22(14-16):1777-1797. doi: 10.1080/15384101.2023.2239620. Epub 2023 Jul 24. Cell Cycle. 2023. PMID: 37486005 Free PMC article.
-
Management of chronic respiratory diseases during viral pandemics: A concise review of guidance and recommendations.J Family Med Prim Care. 2022 Nov;11(11):6633-6639. doi: 10.4103/jfmpc.jfmpc_974_21. Epub 2022 Dec 16. J Family Med Prim Care. 2022. PMID: 36993046 Free PMC article. Review.
References
-
- WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020[Internet]. World Health Organization 2020 [cited 2020 Apr 13]. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at...
-
- WHO characterizes COVID-19 as a pandemic. March 12, 2020[Internet]. World Health Organization 2020 [cited 2020 Apr 13]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-a...
-
- Coronavirus (COVID-19) [Internet]. World Health Organization 2020 [cited 2020 Apr 13]. Available online: https://who.sprinklr.com/
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
