Subcutaneous administration of interferon beta-1a for COVID-19: A non-controlled prospective trial
- PMID: 32544867
- PMCID: PMC7275997
- DOI: 10.1016/j.intimp.2020.106688
Subcutaneous administration of interferon beta-1a for COVID-19: A non-controlled prospective trial
Abstract
Background: Recently, a new coronavirus spreads rapidly throughout the countries and resulted in a worldwide epidemic. Interferons have direct antiviral and immunomodulatory effects. Antiviral effects may include inhibition of viral replication, protein synthesis, virus maturation, or virus release from infected cells. Previous studies have shown that some coronaviruses are susceptible to interferons. The aim of this study was to evaluate the therapeutic effects of IFN-β-1a administration in COVID-19.
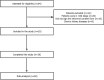
Methods: In this prospective non-controlled trial, 20 patients included. They received IFN-β-1a at a dose of 44 µg subcutaneously every other day up to 10 days. All patients received conventional therapy including Hydroxychloroquine, and lopinavir/ritonavir. Demographic data, clinical symptoms, virological clearance, and imaging findings recorded during the study.
Results: The mean age of the patients was 58.55 ± 13.43 years. Fever resolved in all patients during first seven days. Although other symptoms decreased gradually. Virological clearance results showed a significant decrease within 10 days. Imaging studies showed significant recovery after 14-day period in all patients. The mean time of hospitalization was 16.8 ± 3.4 days. There were no deaths or significant adverse drug reactions in the 14-day period.
Conclusions: Our findings support the use of IFN-β-1a in combination with hydroxychloroquine and lopinavir/ritonavir in the management of COVID-19.
Clinical trial registration number: IRCT20151227025726N12.
Keywords: COVID-19; Coronavirus; Interferon Beta-1a; Pulmonary infection.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
An investigation into the beneficial effects of high-dose interferon beta 1-a, compared to low-dose interferon beta 1-a (the base therapeutic regimen) in moderate to severe COVID-19: A structured summary of a study protocol for a randomized controlled l trial.Trials. 2020 Oct 26;21(1):880. doi: 10.1186/s13063-020-04812-2. Trials. 2020. PMID: 33106183 Free PMC article. Clinical Trial.
-
Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 3;21(1):473. doi: 10.1186/s13063-020-04382-3. Trials. 2020. PMID: 32493468 Free PMC article.
-
A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01061-20. doi: 10.1128/AAC.01061-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32661006 Free PMC article. Clinical Trial.
-
Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2.Biomed Pharmacother. 2020 Nov;131:110668. doi: 10.1016/j.biopha.2020.110668. Epub 2020 Aug 24. Biomed Pharmacother. 2020. PMID: 32861965 Free PMC article. Review.
-
Clinical Trials of Repurposed Antivirals for SARS-CoV-2.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01101-20. doi: 10.1128/AAC.01101-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32631826 Free PMC article. Review.
Cited by
-
Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial.Int Immunopharmacol. 2020 Nov;88:106903. doi: 10.1016/j.intimp.2020.106903. Epub 2020 Aug 24. Int Immunopharmacol. 2020. PMID: 32862111 Free PMC article. Clinical Trial.
-
Immunotherapeutic approaches to curtail COVID-19.Int Immunopharmacol. 2020 Nov;88:106924. doi: 10.1016/j.intimp.2020.106924. Epub 2020 Aug 21. Int Immunopharmacol. 2020. PMID: 32877828 Free PMC article. Review.
-
Is there any potential management against COVID-19? A systematic review and meta-analysis.Daru. 2020 Dec;28(2):765-777. doi: 10.1007/s40199-020-00367-4. Epub 2020 Aug 18. Daru. 2020. PMID: 32812187 Free PMC article.
-
Impact of IFN-β1a in treatment of a COVID-19 patient with beta thalassemia and diabetes mellitus: A case report.Clin Case Rep. 2022 Aug 3;10(8):e6114. doi: 10.1002/ccr3.6114. eCollection 2022 Aug. Clin Case Rep. 2022. PMID: 35937023 Free PMC article.
-
Targeted therapy in Coronavirus disease 2019 (COVID-19): Implication from cell and gene therapy to immunotherapy and vaccine.Int Immunopharmacol. 2022 Oct;111:109161. doi: 10.1016/j.intimp.2022.109161. Epub 2022 Aug 18. Int Immunopharmacol. 2022. PMID: 35998506 Free PMC article. Review.
References
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources