The role of extracellular vesicles in COVID-19 virus infection
- PMID: 32544615
- PMCID: PMC7293471
- DOI: 10.1016/j.meegid.2020.104422
The role of extracellular vesicles in COVID-19 virus infection
Abstract
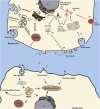
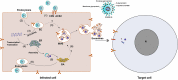
Extracellular vesicles releasing from various types of cells contribute to intercellular communication via delivering bio-molecules like nucleic acids, proteins, and lipids to recipient cells. Exosomes are 30-120 nm extracellular vesicles that participate in several pathological conditions. Virus-infected cells release exosomes that are implicated in infection through transferring viral components such as viral-derived miRNAs and proteins. As well, exosomes contain receptors for viruses that make recipient cells susceptible to virus entry. Since December 2019, SARS-CoV-2 (COVID-19) infection has become a worldwide urgent public health concern. There is currently no vaccine or specific antiviral treatment existing for COVID-19 virus infection. Hence, it is critical to find a safe and effective therapeutic tool to patients with severe COVID-19 virus infection. Extracellular vesicles may contribute to spread this virus as they transfer such receptors as CD9 and ACE2, which make recipient cells susceptible to virus docking. Upon entry, COVID-19 virus may be directed into the exosomal pathway, and its component is packaged into exosomes for secretion. Exosome-based strategies for the treatment of COVID-19 virus infection may include following items: inhibition of exosome biogenesis and uptake, exosome-therapy, exosome-based drug delivery system, and exosome-based vaccine. Mesenchymal stem cells can suppress nonproductive inflammation and improve/repair lung cells including endothelial and alveolar cells, which damaged by COVID-19 virus infection. Understanding molecular mechanisms behind extracellular vesicles related COVID-19 virus infection may provide us with an avenue to identify its entry, replication, spreading, and infection to overcome its adverse effects.
Keywords: COVID-19 virus; Exosomes; Extracellular vesicles; Viral infection.
Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Emerging roles of extracellular vesicles in COVID-19, a double-edged sword?Immunology. 2021 Aug;163(4):416-430. doi: 10.1111/imm.13329. Epub 2021 May 4. Immunology. 2021. PMID: 33742451 Free PMC article. Review.
-
Exosomes, and the potential for exosome-based interventions against COVID-19.Rev Med Virol. 2024 Jul;34(4):e2562. doi: 10.1002/rmv.2562. Rev Med Virol. 2024. PMID: 38924213 Review.
-
Extracellular Vesicles as a Translational Approach for the Treatment of COVID-19 Disease: An Updated Overview.Viruses. 2023 Sep 22;15(10):1976. doi: 10.3390/v15101976. Viruses. 2023. PMID: 37896755 Free PMC article. Review.
-
HACE2-Exosome-Based Nano-Bait for Concurrent SARS-CoV-2 Trapping and Antioxidant Therapy.ACS Appl Mater Interfaces. 2022 Feb 2;14(4):4882-4891. doi: 10.1021/acsami.1c19541. Epub 2022 Jan 22. ACS Appl Mater Interfaces. 2022. PMID: 35067058
-
Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments.Front Immunol. 2021 Jul 28;12:716407. doi: 10.3389/fimmu.2021.716407. eCollection 2021. Front Immunol. 2021. PMID: 34394121 Free PMC article. Review.
Cited by
-
Cardiovascular RNA markers and artificial intelligence may improve COVID-19 outcome: a position paper from the EU-CardioRNA COST Action CA17129.Cardiovasc Res. 2021 Jul 7;117(8):1823-1840. doi: 10.1093/cvr/cvab094. Cardiovasc Res. 2021. PMID: 33839767 Free PMC article.
-
Putative Roles for Peptidylarginine Deiminases in COVID-19.Int J Mol Sci. 2020 Jun 30;21(13):4662. doi: 10.3390/ijms21134662. Int J Mol Sci. 2020. PMID: 32629995 Free PMC article.
-
Single-Cell Gene Expression Analysis Revealed Immune Cell Signatures of Delta COVID-19.Cells. 2022 Sep 21;11(19):2950. doi: 10.3390/cells11192950. Cells. 2022. PMID: 36230912 Free PMC article.
-
Cell-Free Therapies: Novel Approaches for COVID-19.Front Immunol. 2020 Sep 18;11:583017. doi: 10.3389/fimmu.2020.583017. eCollection 2020. Front Immunol. 2020. PMID: 33072130 Free PMC article. Review. No abstract available.
-
Strategies for Small Extracellular Vesicle-Based Cancer Immunotherapy.Research (Wash D C). 2024 Jul 22;7:0421. doi: 10.34133/research.0421. eCollection 2024. Research (Wash D C). 2024. PMID: 39040921 Free PMC article. Review.
References
-
- Abdyazdani N., Nourazarian A., Charoudeh H.N., Kazemi M., Feizy N., Akbarzade M., Mehdizadeh A., Rezaie J., Rahbarghazi R. The role of morphine on rat neural stem cells viability, neuro-angiogenesis and neuro-steroidgenesis properties. Neurosci. Lett. 2017;636:205–212. - PubMed
-
- Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., Sun C., Sylvia S., Rozelle S., Raat H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect. Dis. Poverty. 2020;9:1–12. - PMC - PubMed
-
- Ahmadi M., Rahbarghazi R., Aslani M.R., Shahbazfar A.-A., Kazemi M., Keyhanmanesh R. Bone marrow mesenchymal stem cells and their conditioned media could potentially ameliorate ovalbumin-induced asthmatic changes. Biomed. Pharmacother. 2017;85:28–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

