Rab family of small GTPases: an updated view on their regulation and functions
- PMID: 32542850
- PMCID: PMC7818423
- DOI: 10.1111/febs.15453
Rab family of small GTPases: an updated view on their regulation and functions
Abstract
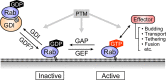
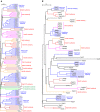
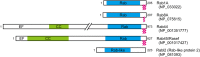
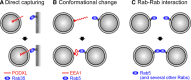
The Rab family of small GTPases regulates intracellular membrane trafficking by orchestrating the biogenesis, transport, tethering, and fusion of membrane-bound organelles and vesicles. Like other small GTPases, Rabs cycle between two states, an active (GTP-loaded) state and an inactive (GDP-loaded) state, and their cycling is catalyzed by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Because an active form of each Rab localizes on a specific organelle (or vesicle) and recruits various effector proteins to facilitate each step of membrane trafficking, knowing when and where Rabs are activated and what effectors Rabs recruit is crucial to understand their functions. Since the discovery of Rabs, they have been regarded as one of the central hubs for membrane trafficking, and numerous biochemical and genetic studies have revealed the mechanisms of Rab functions in recent years. The results of these studies have included the identification and characterization of novel GEFs, GAPs, and effectors, as well as post-translational modifications, for example, phosphorylation, of Rabs. Rab functions beyond the simple effector-recruiting model are also emerging. Furthermore, the recently developed CRISPR/Cas technology has enabled acceleration of knockout analyses in both animals and cultured cells and revealed previously unknown physiological roles of many Rabs. In this review article, we provide the most up-to-date and comprehensive lists of GEFs, GAPs, effectors, and knockout phenotypes of mammalian Rabs and discuss recent findings in regard to their regulation and functions.
Keywords: GAP; GEF; Rab small GTPases; effector; knockout; membrane traffic; organelle; post-translational modification.
© 2020 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Rab regulation by GEFs and GAPs during membrane traffic.Curr Opin Cell Biol. 2019 Aug;59:34-39. doi: 10.1016/j.ceb.2019.03.004. Epub 2019 Apr 10. Curr Opin Cell Biol. 2019. PMID: 30981180 Review.
-
Regulation of membrane traffic by Rab GEF and GAP cascades.Small GTPases. 2016 Oct;7(4):252-256. doi: 10.1080/21541248.2016.1213781. Epub 2016 Jul 18. Small GTPases. 2016. PMID: 27427966 Free PMC article. Review.
-
Multiple Types of Guanine Nucleotide Exchange Factors (GEFs) for Rab Small GTPases.Cell Struct Funct. 2016 Jul 9;41(2):61-79. doi: 10.1247/csf.16008. Epub 2016 May 27. Cell Struct Funct. 2016. PMID: 27246931 Review.
-
Intrinsic tethering activity of endosomal Rab proteins.Nat Struct Mol Biol. 2011 Dec 11;19(1):40-7. doi: 10.1038/nsmb.2162. Nat Struct Mol Biol. 2011. PMID: 22157956 Free PMC article.
-
Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases.J Biol Chem. 2013 Oct 4;288(40):28704-12. doi: 10.1074/jbc.M113.488213. Epub 2013 Aug 26. J Biol Chem. 2013. PMID: 23979137 Free PMC article.
Cited by
-
The progress in C9orf72 research: ALS/FTD pathogenesis, functions and structure.Small GTPases. 2022 Jan;13(1):56-76. doi: 10.1080/21541248.2021.1892443. Epub 2021 Mar 5. Small GTPases. 2022. PMID: 33663328 Free PMC article. Review.
-
De novo variants in DENND5B cause a neurodevelopmental disorder.Am J Hum Genet. 2024 Mar 7;111(3):529-543. doi: 10.1016/j.ajhg.2024.02.001. Epub 2024 Feb 21. Am J Hum Genet. 2024. PMID: 38387458 Free PMC article.
-
RAB31 drives extracellular vesicle fusion and cancer-associated fibroblast formation leading to oxaliplatin resistance in colorectal cancer.J Extracell Biol. 2024 Feb 23;3(2):e141. doi: 10.1002/jex2.141. eCollection 2024 Feb. J Extracell Biol. 2024. PMID: 38939899 Free PMC article.
-
Two roads diverged in a cell: insights from differential exosome regulation in polarized cells.Front Cell Dev Biol. 2024 Sep 2;12:1451988. doi: 10.3389/fcell.2024.1451988. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39286483 Free PMC article. Review.
-
Trafficking in blood vessel development.Angiogenesis. 2022 Aug;25(3):291-305. doi: 10.1007/s10456-022-09838-5. Epub 2022 Apr 21. Angiogenesis. 2022. PMID: 35449244 Free PMC article. Review.
References
-
- Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10, 513–525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

