Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial
- PMID: 32492084
- PMCID: PMC7270883
- DOI: 10.1001/jama.2020.10044
Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial
Erratum in
-
Correction to Data in Trial of Convalescent Plasma Treatment for COVID-19.JAMA. 2020 Aug 4;324(5):519. doi: 10.1001/jama.2020.13216. JAMA. 2020. PMID: 32749474 Free PMC article. No abstract available.
Abstract
Importance: Convalescent plasma is a potential therapeutic option for patients with coronavirus disease 2019 (COVID-19), but further data from randomized clinical trials are needed.
Objective: To evaluate the efficacy and adverse effects of convalescent plasma therapy for patients with COVID-19.
Design, setting, and participants: Open-label, multicenter, randomized clinical trial performed in 7 medical centers in Wuhan, China, from February 14, 2020, to April 1, 2020, with final follow-up April 28, 2020. The trial included 103 participants with laboratory-confirmed COVID-19 that was severe (respiratory distress and/or hypoxemia) or life-threatening (shock, organ failure, or requiring mechanical ventilation). The trial was terminated early after 103 of a planned 200 patients were enrolled.
Intervention: Convalescent plasma in addition to standard treatment (n = 52) vs standard treatment alone (control) (n = 51), stratified by disease severity.
Main outcomes and measures: Primary outcome was time to clinical improvement within 28 days, defined as patient discharged alive or reduction of 2 points on a 6-point disease severity scale (ranging from 1 [discharge] to 6 [death]). Secondary outcomes included 28-day mortality, time to discharge, and the rate of viral polymerase chain reaction (PCR) results turned from positive at baseline to negative at up to 72 hours.
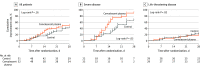
Results: Of 103 patients who were randomized (median age, 70 years; 60 [58.3%] male), 101 (98.1%) completed the trial. Clinical improvement occurred within 28 days in 51.9% (27/52) of the convalescent plasma group vs 43.1% (22/51) in the control group (difference, 8.8% [95% CI, -10.4% to 28.0%]; hazard ratio [HR], 1.40 [95% CI, 0.79-2.49]; P = .26). Among those with severe disease, the primary outcome occurred in 91.3% (21/23) of the convalescent plasma group vs 68.2% (15/22) of the control group (HR, 2.15 [95% CI, 1.07-4.32]; P = .03); among those with life-threatening disease the primary outcome occurred in 20.7% (6/29) of the convalescent plasma group vs 24.1% (7/29) of the control group (HR, 0.88 [95% CI, 0.30-2.63]; P = .83) (P for interaction = .17). There was no significant difference in 28-day mortality (15.7% vs 24.0%; OR, 0.59 [95% CI, 0.22-1.59]; P = .30) or time from randomization to discharge (51.0% vs 36.0% discharged by day 28; HR, 1.61 [95% CI, 0.88-2.95]; P = .12). Convalescent plasma treatment was associated with a negative conversion rate of viral PCR at 72 hours in 87.2% of the convalescent plasma group vs 37.5% of the control group (OR, 11.39 [95% CI, 3.91-33.18]; P < .001). Two patients in the convalescent plasma group experienced adverse events within hours after transfusion that improved with supportive care.
Conclusion and relevance: Among patients with severe or life-threatening COVID-19, convalescent plasma therapy added to standard treatment, compared with standard treatment alone, did not result in a statistically significant improvement in time to clinical improvement within 28 days. Interpretation is limited by early termination of the trial, which may have been underpowered to detect a clinically important difference.
Trial registration: Chinese Clinical Trial Registry: ChiCTR2000029757.
Conflict of interest statement
Figures

Comment in
-
A Randomized Trial of Convalescent Plasma for COVID-19-Potentially Hopeful Signals.JAMA. 2020 Aug 4;324(5):455-457. doi: 10.1001/jama.2020.10218. JAMA. 2020. PMID: 32492105 No abstract available.
-
Errors in Trial of Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19.JAMA. 2020 Aug 4;324(5):518-519. doi: 10.1001/jama.2020.12607. JAMA. 2020. PMID: 32749486 No abstract available.
Similar articles
-
Evaluating the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 8;21(1):499. doi: 10.1186/s13063-020-04422-y. Trials. 2020. PMID: 32513308 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Jul 10;7(7):CD013600. doi: 10.1002/14651858.CD013600.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3 PMID: 32648959 Free PMC article. Updated.
-
A Randomized Open label Phase-II Clinical Trial with or without Infusion of Plasma from Subjects after Convalescence of SARS-CoV-2 Infection in High-Risk Patients with Confirmed Severe SARS-CoV-2 Disease (RECOVER): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 6;21(1):828. doi: 10.1186/s13063-020-04735-y. Trials. 2020. PMID: 33023671 Free PMC article.
-
Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks.Clin Microbiol Infect. 2020 Oct;26(10):1436-1446. doi: 10.1016/j.cmi.2020.08.005. Epub 2020 Aug 11. Clin Microbiol Infect. 2020. PMID: 32791241 Free PMC article. Review.
-
Convalescent plasma for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2023 Feb 1;2(2):CD013600. doi: 10.1002/14651858.CD013600.pub5. Cochrane Database Syst Rev. 2023. Update in: Cochrane Database Syst Rev. 2023 May 10;5:CD013600. doi: 10.1002/14651858.CD013600.pub6 PMID: 36734509 Free PMC article. Updated. Review.
Cited by
-
Development and application of therapeutic antibodies against COVID-19.Int J Biol Sci. 2021 Apr 10;17(6):1486-1496. doi: 10.7150/ijbs.59149. eCollection 2021. Int J Biol Sci. 2021. PMID: 33907512 Free PMC article. Review.
-
Convalescent plasma to treat critically ill patients with COVID-19: framing the need for randomised clinical trials.Crit Care. 2020 Jul 20;24(1):449. doi: 10.1186/s13054-020-03163-3. Crit Care. 2020. PMID: 32690059 Free PMC article. No abstract available.
-
Cardiovascular RNA markers and artificial intelligence may improve COVID-19 outcome: a position paper from the EU-CardioRNA COST Action CA17129.Cardiovasc Res. 2021 Jul 7;117(8):1823-1840. doi: 10.1093/cvr/cvab094. Cardiovasc Res. 2021. PMID: 33839767 Free PMC article.
-
Convalescent Blood: Current Perspective on the Efficacy of a Legacy Approach in COVID-19 Treatment.Blood Purif. 2022;51(1):1-14. doi: 10.1159/000513164. Epub 2021 Mar 31. Blood Purif. 2022. PMID: 33789273 Free PMC article. Review.
-
The Principles of Antibody Therapy for Infectious Diseases with Relevance for COVID-19.mBio. 2021 Mar 2;12(2):e03372-20. doi: 10.1128/mBio.03372-20. mBio. 2021. PMID: 33653885 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

