Protrudin-mediated ER-endosome contact sites promote MT1-MMP exocytosis and cell invasion
- PMID: 32479595
- PMCID: PMC7401796
- DOI: 10.1083/jcb.202003063
Protrudin-mediated ER-endosome contact sites promote MT1-MMP exocytosis and cell invasion
Abstract
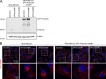
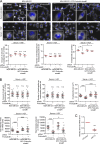
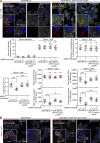
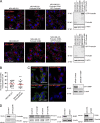
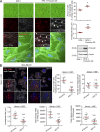
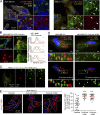
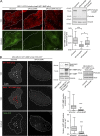
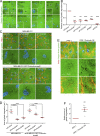
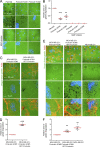
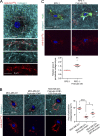
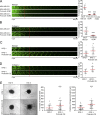
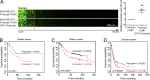
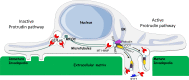
Cancer cells break tissue barriers by use of small actin-rich membrane protrusions called invadopodia. Complete invadopodia maturation depends on protrusion outgrowth and the targeted delivery of the matrix metalloproteinase MT1-MMP via endosomal transport by mechanisms that are not known. Here, we show that the ER protein Protrudin orchestrates invadopodia maturation and function. Protrudin formed contact sites with MT1-MMP-positive endosomes that contained the RAB7-binding Kinesin-1 adaptor FYCO1, and depletion of RAB7, FYCO1, or Protrudin inhibited MT1-MMP-dependent extracellular matrix degradation and cancer cell invasion by preventing anterograde translocation and exocytosis of MT1-MMP. Moreover, when endosome translocation or exocytosis was inhibited by depletion of Protrudin or Synaptotagmin VII, respectively, invadopodia were unable to expand and elongate. Conversely, when Protrudin was overexpressed, noncancerous cells developed prominent invadopodia-like protrusions and showed increased matrix degradation and invasion. Thus, Protrudin-mediated ER-endosome contact sites promote cell invasion by facilitating translocation of MT1-MMP-laden endosomes to the plasma membrane, enabling both invadopodia outgrowth and MT1-MMP exocytosis.
© 2020 Pedersen et al.
Figures


Comment in
-
Protrudin in protrudinG invadopodia: Membrane contact sites and cell invasion.J Cell Biol. 2020 Aug 3;219(8):e202006146. doi: 10.1083/jcb.202006146. J Cell Biol. 2020. PMID: 32692799 Free PMC article.
Similar articles
-
Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth.Nature. 2015 Apr 9;520(7546):234-8. doi: 10.1038/nature14359. Nature. 2015. PMID: 25855459
-
MT1-MMP targeting to endolysosomes is mediated by upregulation of flotillins.J Cell Sci. 2018 Sep 5;131(17):jcs218925. doi: 10.1242/jcs.218925. J Cell Sci. 2018. PMID: 30111578
-
Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia.J Cell Biol. 2013 Dec 23;203(6):1063-79. doi: 10.1083/jcb.201306162. J Cell Biol. 2013. PMID: 24344185 Free PMC article.
-
Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia.J Cell Sci. 2009 Sep 1;122(Pt 17):3015-24. doi: 10.1242/jcs.034561. J Cell Sci. 2009. PMID: 19692588 Review.
-
Cellular and Molecular Mechanisms of MT1-MMP-Dependent Cancer Cell Invasion.Annu Rev Cell Dev Biol. 2016 Oct 6;32:555-576. doi: 10.1146/annurev-cellbio-111315-125227. Epub 2016 Aug 8. Annu Rev Cell Dev Biol. 2016. PMID: 27501444 Review.
Cited by
-
Protein dynamics at invadopodia control invasion-migration transitions in melanoma cells.Cell Death Dis. 2023 Mar 11;14(3):190. doi: 10.1038/s41419-023-05704-4. Cell Death Dis. 2023. PMID: 36899008 Free PMC article.
-
Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression.Int J Mol Sci. 2021 Dec 23;23(1):146. doi: 10.3390/ijms23010146. Int J Mol Sci. 2021. PMID: 35008569 Free PMC article. Review.
-
A MAP1B-cortactin-Tks5 axis regulates TNBC invasion and tumorigenesis.J Cell Biol. 2024 Mar 4;223(3):e202303102. doi: 10.1083/jcb.202303102. Epub 2024 Feb 14. J Cell Biol. 2024. PMID: 38353696 Free PMC article.
-
Transcription factor EB regulates phosphatidylinositol-3-phosphate levels that control lysosome positioning in the bladder cancer model.Commun Biol. 2023 Jan 28;6(1):114. doi: 10.1038/s42003-023-04501-1. Commun Biol. 2023. PMID: 36709383 Free PMC article.
-
The RUFYs, a Family of Effector Proteins Involved in Intracellular Trafficking and Cytoskeleton Dynamics.Front Cell Dev Biol. 2020 Aug 11;8:779. doi: 10.3389/fcell.2020.00779. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850870 Free PMC article. Review.
References
-
- Artym V.V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K.M., and Mueller S.C.. 2006. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66:3034–3043. 10.1158/0008-5472.CAN-05-2177 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

