The Nuclear Pore Complex: A Target for NS3 Protease of Dengue and Zika Viruses
- PMID: 32466480
- PMCID: PMC7354628
- DOI: 10.3390/v12060583
The Nuclear Pore Complex: A Target for NS3 Protease of Dengue and Zika Viruses
Abstract
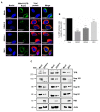
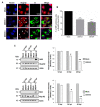
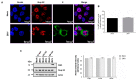
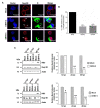
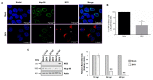
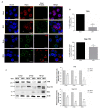
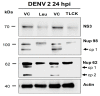
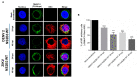
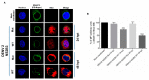
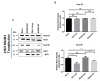
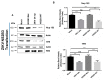
During flavivirus infection, some viral proteins move to the nucleus and cellular components are relocated from the nucleus to the cytoplasm. Thus, the integrity of the main regulator of the nuclear-cytoplasmic transport, the nuclear pore complex (NPC), was evaluated during infection with dengue virus (DENV) and Zika virus (ZIKV). We found that while during DENV infection the integrity and distribution of at least three nucleoporins (Nup), Nup153, Nup98, and Nup62 were altered, during ZIKV infection, the integrity of TPR, Nup153, and Nup98 were modified. In this work, several lines of evidence indicate that the viral serine protease NS2B3 is involved in Nups cleavage. First, the serine protease inhibitors, TLCK and Leupeptin, prevented Nup98 and Nup62 cleavage. Second, the transfection of DENV and ZIKV NS2B3 protease was sufficient to inhibit the nuclear ring recognition detected in mock-infected cells with the Mab414 antibody. Third, the mutant but not the active (WT) protease was unable to cleave Nups in transfected cells. Thus, here we describe for the first time that the NS3 protein from flavivirus plays novel functions hijacking the nuclear pore complex, the main controller of the nuclear-cytoplasmic transport.
Keywords: NS3; dengue; nuclear pore complex; nucleus; zika.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dengue and Zika viruses subvert reticulophagy by NS2B3-mediated cleavage of FAM134B.Autophagy. 2017 Feb;13(2):322-332. doi: 10.1080/15548627.2016.1265192. Epub 2017 Jan 19. Autophagy. 2017. PMID: 28102736 Free PMC article.
-
Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1.J Virol. 2023 Jan 31;97(1):e0177322. doi: 10.1128/jvi.01773-22. Epub 2022 Dec 8. J Virol. 2023. PMID: 36475764 Free PMC article.
-
BGLF4 kinase modulates the structure and transport preference of the nuclear pore complex to facilitate nuclear import of Epstein-Barr virus lytic proteins.J Virol. 2015 Feb;89(3):1703-18. doi: 10.1128/JVI.02880-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410863 Free PMC article.
-
Exploiting the unique features of Zika and Dengue proteases for inhibitor design.Biochimie. 2019 Nov;166:132-141. doi: 10.1016/j.biochi.2019.05.004. Epub 2019 May 9. Biochimie. 2019. PMID: 31077760 Review.
-
Immune Response to Dengue and Zika.Annu Rev Immunol. 2018 Apr 26;36:279-308. doi: 10.1146/annurev-immunol-042617-053142. Epub 2018 Jan 18. Annu Rev Immunol. 2018. PMID: 29345964 Free PMC article. Review.
Cited by
-
Flavivirus infections induce a Golgi stress response in vertebrate and mosquito cells.Sci Rep. 2021 Dec 6;11(1):23489. doi: 10.1038/s41598-021-02929-1. Sci Rep. 2021. PMID: 34873243 Free PMC article.
-
Transcriptomic profiling implicates PAF1 in both active and repressive immune regulatory networks.BMC Genomics. 2022 Nov 30;23(1):787. doi: 10.1186/s12864-022-09013-6. BMC Genomics. 2022. PMID: 36451099 Free PMC article.
-
Differential localization of dengue virus protease affects cell homeostasis and triggers to thrombocytopenia.iScience. 2023 Jun 5;26(7):107024. doi: 10.1016/j.isci.2023.107024. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534186 Free PMC article.
-
The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell-Cell Communication: New Insights on Virus-Host Interactions.Viruses. 2020 Jul 16;12(7):765. doi: 10.3390/v12070765. Viruses. 2020. PMID: 32708685 Free PMC article. Review.
-
The Role of Protein Disorder in Nuclear Transport and in Its Subversion by Viruses.Cells. 2020 Dec 10;9(12):2654. doi: 10.3390/cells9122654. Cells. 2020. PMID: 33321790 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

