Remdesivir for the Treatment of Covid-19 - Final Report
- PMID: 32445440
- PMCID: PMC7262788
- DOI: 10.1056/NEJMoa2007764
Remdesivir for the Treatment of Covid-19 - Final Report
Abstract
Background: Although several therapeutic agents have been evaluated for the treatment of coronavirus disease 2019 (Covid-19), no antiviral agents have yet been shown to be efficacious.
Methods: We conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection. Patients were randomly assigned to receive either remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) or placebo for up to 10 days. The primary outcome was the time to recovery, defined by either discharge from the hospital or hospitalization for infection-control purposes only.
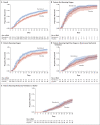
Results: A total of 1062 patients underwent randomization (with 541 assigned to remdesivir and 521 to placebo). Those who received remdesivir had a median recovery time of 10 days (95% confidence interval [CI], 9 to 11), as compared with 15 days (95% CI, 13 to 18) among those who received placebo (rate ratio for recovery, 1.29; 95% CI, 1.12 to 1.49; P<0.001, by a log-rank test). In an analysis that used a proportional-odds model with an eight-category ordinal scale, the patients who received remdesivir were found to be more likely than those who received placebo to have clinical improvement at day 15 (odds ratio, 1.5; 95% CI, 1.2 to 1.9, after adjustment for actual disease severity). The Kaplan-Meier estimates of mortality were 6.7% with remdesivir and 11.9% with placebo by day 15 and 11.4% with remdesivir and 15.2% with placebo by day 29 (hazard ratio, 0.73; 95% CI, 0.52 to 1.03). Serious adverse events were reported in 131 of the 532 patients who received remdesivir (24.6%) and in 163 of the 516 patients who received placebo (31.6%).
Conclusions: Our data show that remdesivir was superior to placebo in shortening the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection. (Funded by the National Institute of Allergy and Infectious Diseases and others; ACTT-1 ClinicalTrials.gov number, NCT04280705.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
Remdesivir - An Important First Step.N Engl J Med. 2020 Nov 5;383(19):1886-1887. doi: 10.1056/NEJMe2018715. Epub 2020 May 27. N Engl J Med. 2020. PMID: 32459913 Free PMC article. No abstract available.
-
[Remdesivir til behandling af COVID-19-pneumoni].Ugeskr Laeger. 2020 Jun 8;182(24):V205031. Ugeskr Laeger. 2020. PMID: 32515337 Danish. No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):992. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649074 No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):992-993. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649075 No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):993. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649076 No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):993-994. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649077 No abstract available.
-
The Adaptive COVID-19 Treatment Trial-1 (ACTT-1) in a real-world population: a comparative observational study.Crit Care. 2020 Dec 7;24(1):677. doi: 10.1186/s13054-020-03406-3. Crit Care. 2020. PMID: 33287853 Free PMC article. No abstract available.
Similar articles
-
Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2020 Sep 15;324(11):1048-1057. doi: 10.1001/jama.2020.16349. JAMA. 2020. PMID: 32821939 Free PMC article. Clinical Trial.
-
Remdesivir for 5 or 10 Days in Patients with Severe Covid-19.N Engl J Med. 2020 Nov 5;383(19):1827-1837. doi: 10.1056/NEJMoa2015301. Epub 2020 May 27. N Engl J Med. 2020. PMID: 32459919 Free PMC article. Clinical Trial.
-
Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial.Lancet. 2020 May 16;395(10236):1569-1578. doi: 10.1016/S0140-6736(20)31022-9. Epub 2020 Apr 29. Lancet. 2020. PMID: 32423584 Free PMC article. Clinical Trial.
-
Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies.Diabetes Metab Syndr. 2020 Jul-Aug;14(4):641-648. doi: 10.1016/j.dsx.2020.05.018. Epub 2020 May 12. Diabetes Metab Syndr. 2020. PMID: 32428865 Free PMC article. Review.
-
Remdesivir against COVID-19 and Other Viral Diseases.Clin Microbiol Rev. 2020 Oct 14;34(1):e00162-20. doi: 10.1128/CMR.00162-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33055231 Free PMC article. Review.
Cited by
-
Multidrug Combinations against SARS-CoV-2 Using GS-441524 or Ivermectin with Molnupiravir and/or Nirmatrelvir in Reconstituted Human Nasal Airway Epithelia.Pharmaceutics. 2024 Sep 27;16(10):1262. doi: 10.3390/pharmaceutics16101262. Pharmaceutics. 2024. PMID: 39458594 Free PMC article.
-
Vaccines and drugs under clinical trials for prevention and treatment of COVID-19.Virusdisease. 2021 Mar;32(1):13-19. doi: 10.1007/s13337-020-00650-7. Epub 2021 Mar 22. Virusdisease. 2021. PMID: 33778130 Free PMC article. Review.
-
2021 Acute Respiratory Distress Syndrome Update, With Coronavirus Disease 2019 Focus.J Cardiothorac Vasc Anesth. 2022 Apr;36(4):1188-1195. doi: 10.1053/j.jvca.2021.02.053. Epub 2021 Feb 27. J Cardiothorac Vasc Anesth. 2022. PMID: 33781671 Free PMC article. Review.
-
Protocol for assessing and predicting acute respiratory decline in hospitalized patients.STAR Protoc. 2021 Jun 18;2(2):100545. doi: 10.1016/j.xpro.2021.100545. Epub 2021 May 19. STAR Protoc. 2021. PMID: 34027496 Free PMC article.
-
Fewer COVID-19 Neurological Complications with Dexamethasone and Remdesivir.Ann Neurol. 2023 Jan;93(1):88-102. doi: 10.1002/ana.26536. Epub 2022 Nov 9. Ann Neurol. 2023. PMID: 36261315 Free PMC article.
References
-
- Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3(4):e208857-e208857. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1AI148576/US/United States/United States
- I01 BX003714/BX/BLRD VA/United States
- UM1AI148573/US/United States/United States
- UM1AI148685/US/United States/United States
- UM1AI148452/US/United States/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- UM1 AI148573/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- HHSN261201500003I/CA/NCI NIH HHS/United States
- HHSN261200800001E/US/United States/United States
- R38 AI140299/AI/NIAID NIH HHS/United States
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- UM1 AI148575/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1AI148575/US/United States/United States
- HHSN261201500003C/CA/NCI NIH HHS/United States
- UM1AI148684/US/United States/United States
- MC_UU_12023/22/MRC_/Medical Research Council/United Kingdom
- UM1AI148689/US/United States/United States
- UM1 AI148452/AI/NIAID NIH HHS/United States
- HHSN261201500003I/US/United States/United States
- UM1AI148450/US/United States/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
