Dietary interventions for multiple sclerosis-related outcomes
- PMID: 32428983
- PMCID: PMC7388136
- DOI: 10.1002/14651858.CD004192.pub4
Dietary interventions for multiple sclerosis-related outcomes
Abstract
Background: Multiple sclerosis (MS) is a common demyelinating disease of the central nervous system. Although the exact pathogenesis remains unknown, the leading theory is that it results from immune system dysregulation. Approved disease-modifying therapy appears to modulate the immune system to improve MS-related outcomes. There is substantial interest in the ability of dietary interventions to influence MS-related outcomes. This is an update of the Cochrane Review 'Dietary interventions for multiple sclerosis' (Farinotti 2003; Farinotti 2007; Farinotti 2012).
Objectives: To assess the effects of dietary interventions (including dietary plans with recommendations for specific whole foods, macronutrients, and natural health products) compared to placebo or another intervention on health outcomes (including MS-related outcomes and serious adverse events) in people with MS.
Search methods: On 30 May 2019, we searched CENTRAL, MEDLINE, Embase, and Web of Science. We also searched ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform (ICTRP), and Networked Digital Library of Theses and Dissertations (NDLTD). We checked reference lists in identified trials and requested information from trial authors to identify any additional published or unpublished data.
Selection criteria: We included any randomized controlled trial (RCT) or controlled clinical trial (CCT) examining the effect of a dietary intervention versus placebo or another intervention among participants with MS on MS-related outcomes, including relapses, disability progression, and magnetic resonance imaging (MRI) measures.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Planned primary outcomes were number of participants experiencing relapse and change in disability progression, according to a validated disability scale at the last reported follow-up. Secondary outcomes included MRI activity, safety, and patient-reported outcomes. We entered and analysed data in Review Manager 5.
Main results: We found 41 full-text articles examining 30 trials following full-text review. Participants were adults with MS, defined by established criteria, presenting to MS clinics in Europe, North America, and the Middle East. Study design varied considerably, although all trials had at least one methodological issue leading to unknown or high risk of bias. Trials examined: supplementation to increase polyunsaturated fatty acids (PUFAs) (11 trials); a variety of antioxidant supplements (10 trials); dietary programmes (3 trials); and other dietary supplements (e.g. acetyl L-carnitine, biotin, creatine, palmitoylethanolamide, probiotic, riboflavin) (6 trials). In three trials comparing PUFAs with monounsaturated fatty acids (MUFAs), the evidence was very uncertain concerning difference in relapses (risk ratio (RR) 1.02, 95% confidence interval (CI) 0.88 to 1.20; 3 studies, 217 participants; 75% in the PUFA group versus 74% in the MUFA group; very low-certainty evidence). Among four trials comparing PUFAs with MUFAs, there may be little to no difference in global impression of deterioration (RR 0.85, 95% CI 0.71 to 1.03; 4 studies, 542 participants; 40% in the PUFA group versus 47% in the MUFA group; low-certainty evidence). In two trials comparing PUFAs with MUFAs (102 participants), there was very low-certainty evidence for change in disability progression. None of the PUFA versus MUFA trials examined MRI outcomes. In one trial comparing PUFAs with MUFAs (40 participants), there were no serious adverse events; based on low-certainty evidence. In two trials comparing different PUFAs (omega-3 versus omega-6), there may be little to no difference in relapses (RR 1.02, 95% CI 0.62 to 1.66; 2 studies, 129 participants; 30% in the omega-3 versus 29% in the omega-6 group; low-certainty evidence). Among three trials comparing omega-3 with omega-6, there may be little to no difference in change in disability progression, measured as mean change in Expanded Disability Status Scale (EDSS) (mean difference (MD) 0.00, 95% CI -0.30 to 0.30; 3 studies, 166 participants; low-certainty evidence). In one trial comparing omega-3 with omega-6, there was likely no difference in global impression of deterioration (RR 0.99, 95% CI 0.51 to 1.91; 1 study, 86 participants; 29% in omega-3 versus 29% in omega-6 group; moderate-certainty evidence). In one trial comparing omega-3 with omega-6 (86 participants), there was likely no difference in number of new T1- weighted gadolinium-enhancing lesions, based on moderate-certainty evidence. In four trials comparing omega-3 with omega-6, there may be little to no difference in serious adverse events (RR 1.12, 95% CI 0.38 to 3.31; 4 studies, 230 participants; 6% in omega-3 versus 5% in omega-6 group; low-certainty evidence). In four trials examining antioxidant supplementation with placebo, there may be little to no difference in relapses (RR 0.98, 95% CI 0.59 to 1.64; 4 studies, 345 participants; 17% in the antioxidant group versus 17% in the placebo group; low-certainty evidence). In six trials examining antioxidant supplementation with placebo, the evidence was very uncertain concerning change in disability progression, measured as mean change of EDSS (MD -0.19, 95% CI -0.49 to 0.11; 6 studies, 490 participants; very low-certainty evidence). In two trials examining antioxidant supplementation with placebo, there may be little to no difference in global impression of deterioration (RR 0.99, 95% 0.50 to 1.93; 2 studies, 190 participants; 15% in the antioxidant group versus 15% in the placebo group; low-certainty evidence). In two trials examining antioxidant supplementation with placebo, the evidence was very uncertain concerning difference in gadolinium-enhancing lesions (RR 0.67, 95% CI 0.09 to 4.88; 2 studies, 131 participants; 11% in the antioxidant group versus 16% in the placebo group; very low-certainty evidence). In three trials examining antioxidant supplementation versus placebo, there may be little to no difference in serious adverse events (RR. 0.72, 95% CI 0.17 to 3.08; 3 studies, 222 participants; 3% in the antioxidant group versus 4% in the placebo group; low-certainty evidence).
Authors' conclusions: There are a variety of controlled trials addressing the effects of dietary interventions for MS with substantial variation in active treatment, comparator, and outcomes of interest. PUFA administration may not differ when compared to alternatives with regards to relapse rate, disability worsening, or overall clinical status in people with MS, but evidence is uncertain. Similarly, at present, there is insufficient evidence to determine whether supplementation with antioxidants or other dietary interventions have any impact on MS-related outcomes.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Natalie E Parks has provided consulting services to Biogen, EMD Serono, Roche, and Sanofi Genzyme. She has accepted funds from Biogen and Roche for travel to a scientific conference. She has acted as site sub‐investigator for clinical trials for Biogen, MedDay, Sanofi Genzyme, and Roche. She is the recipient of a Killam Predoctoral Scholarship, Nova Scotia Graduate Scholarship, and Dalhousie Medical Research Foundation Multiple Sclerosis Graduate Studentship.
Caitlin S Jackson‐Tarlton: nothing to declare Laura Vacchi: nothing to declare Roah Merdad: nothing to declare Bradley C Johnston: As part of his recruitment to Texas A&M University, BCJ received a start‐up grant from Texas A&M AgriLife Research to fund investigator‐initiated research related to saturated and polyunsaturated fats. The grant was from Texas A&M AgriLife institutional funds from interest and investment earnings, not a sponsoring organization, industry, or company.
Figures
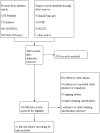
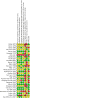
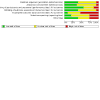























Update of
-
Dietary interventions for multiple sclerosis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD004192. doi: 10.1002/14651858.CD004192.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2020 May 19;5:CD004192. doi: 10.1002/14651858.CD004192.pub4 PMID: 23235605 Updated. Review.
Comment in
-
Dietary interventions for multiple sclerosis-related outcomes: Summary of a cochrane review.Explore (NY). 2022 Mar-Apr;18(2):252-253. doi: 10.1016/j.explore.2021.12.007. Epub 2022 Jan 2. Explore (NY). 2022. PMID: 35031230 No abstract available.
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Siponimod for multiple sclerosis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD013647. doi: 10.1002/14651858.CD013647.pub2. Cochrane Database Syst Rev. 2021. PMID: 34783010 Free PMC article. Review.
-
Dietary interventions for multiple sclerosis.Cochrane Database Syst Rev. 2012 Dec 12;12:CD004192. doi: 10.1002/14651858.CD004192.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2020 May 19;5:CD004192. doi: 10.1002/14651858.CD004192.pub4 PMID: 23235605 Updated. Review.
-
Dietary interventions for multiple sclerosis.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD004192. doi: 10.1002/14651858.CD004192.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Dec 12;12:CD004192. doi: 10.1002/14651858.CD004192.pub3 PMID: 17253500 Updated. Review.
-
Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease.Cochrane Database Syst Rev. 2019 Dec 18;12(12):CD011016. doi: 10.1002/14651858.CD011016.pub2. Cochrane Database Syst Rev. 2019. PMID: 31847055 Free PMC article.
Cited by
-
Role of Ketogenic Diets in Multiple Sclerosis and Related Animal Models: An Updated Review.Adv Nutr. 2022 Oct 2;13(5):2002-2014. doi: 10.1093/advances/nmac065. Adv Nutr. 2022. PMID: 35679067 Free PMC article. Review.
-
Gut flora in multiple sclerosis: implications for pathogenesis and treatment.Neural Regen Res. 2024 Jul 1;19(7):1480-1488. doi: 10.4103/1673-5374.387974. Epub 2023 Nov 13. Neural Regen Res. 2024. PMID: 38051890 Free PMC article.
-
Blood-based targeted metabolipidomics reveals altered omega fatty acid-derived lipid mediators in relapsing-remitting multiple sclerosis patients.bioRxiv [Preprint]. 2024 Jan 6:2024.01.04.574253. doi: 10.1101/2024.01.04.574253. bioRxiv. 2024. PMID: 38260401 Free PMC article. Preprint.
-
Exploring the Therapeutic Potential: Bioactive Molecules and Dietary Interventions in Multiple Sclerosis Management.Curr Issues Mol Biol. 2024 Jun 3;46(6):5595-5613. doi: 10.3390/cimb46060335. Curr Issues Mol Biol. 2024. PMID: 38921006 Free PMC article. Review.
-
Using Online 24-h Dietary Methodology to Validate the Psychometric Properties of a Dietary Scoring Tool with an International Sample of Adults Living with Multiple Sclerosis.Nutrients. 2022 Oct 31;14(21):4568. doi: 10.3390/nu14214568. Nutrients. 2022. PMID: 36364830 Free PMC article.
References
References to studies included in this review
Bates 1977 {published data only}
Bates 1978 {published data only}
-
- Shaw D. Trial of polyunsaturated fatty acids in multiple sclerosis [abstract]. Irish Journal of Medical Science 1978;147(3):118. [CN-00257675]
Bates 1989 {published data only}
-
- Bates D, Cartlidge NE, French JM. Results of a trial of N-3 polyunsaturated fatty acids in the treatment of multiple sclerosis [abstract]. Irish Journal of Medical Science 1988;157(8):277.
Bitarafan 2015 {published data only}
-
- Bitarafan S, Saboor-Yaraghi A, Sahraian MA, Nafissi S, Togha M, Beladi Moghadam N, et al. Impact of vitamin A supplementation on disease progression in patients with multiple sclerosis. Archives of Iranian Medicine 2015;18(7):435-40. [PMID: ] - PubMed
-
- Bitarafan S, Saboor-Yaraghi A, Sahraian MA, Soltani D, Nafissi S, Togha M, et al. Effect of Vitamin A supplementation on fatigue and depression in multiple sclerosis patients: A double-blind placebo-controlled clinical trial. Iranian Journal of Allergy, Asthma, and Immunology 2016;15(1):13-19. [PMID: ] - PubMed
-
- Harrirchian MH, Mohammadzadeh Honarvar N, Koohdani F, Bitarafan S, Siassi F, Jafarirad S, et al. The effect of vitamin A supplementation on disease progression, cytokine levels and gene expression in multiple sclerotic patients: Study protocol for a randomized controlled trial. Acta Medica Iranica 2014;52(2):94-100. [PMID: ] - PubMed
Gallien 2014 {published and unpublished data}
-
- Gallien P, Amarenco G, Benoit N, Bonniaud V, Donzé C, Kerdraon J, et al. Cranberry versus placebo in the prevention of urinary infections in multiple sclerosis: A multicenter, randomized, placebo-controlled, double-blind trial. Multiple Sclerosis Journal 2014;20(9):1252-9. [PMID: ] - PubMed
Gonsette 2010 {published data only}
-
- Gonsette RE, Sindic C, D'hooghe MB, De Deyn PP, Medaer R, Michotte A, et al. Boosting endogenous neuroprotection in multiple sclerosis: the association of inosine and interferon beta in relapsing remitting multiple sclerosis (ASIIMS) trial. Multiple Sclerosis Journal 2010;16:455-62. [PMID: ] - PubMed
-
- Gonsette RF, Sindic C, D'Hooghe MB, Medaer R, Michotte A, De Deyn P, et al. Association of interferon beta and inosine in relapsing-remitting multiple sclerosis (ASIIMS): a multi-centre, randomized, double-blind, placebo-controlled phase II (proof of concept) trial in 157 patients [abstract]. Multiple Sclerosis Journal 2008;14:S44.
Irish 2017 {published data only}
-
- Irish AK, Erickson CM, Wahls TL, Snetselaar LG, Darling WG. Randomized control trial evaluation of a modified paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: A pilot study. Degenerative Neurological and Neuromuscular Disease 2017;7:1-18. [PMID: ] - PMC - PubMed
Khalili 2012 {published data only}
-
- Khalili M, Eskandarai G, Ghajarzadeh M, Azimi A, Eghtesadi S, Sahraian MA, et al. Lipoic acid and multiple sclerosis: A randomized controlled clinical trial. Current Topics in Nutraceutical Research 2012;10:95-100.
Khalili 2014 {published data only}
-
- Khalili M, Azimi A, Izadi V, Eghtesadi S, Mirshafiey A, Sahraian MA, et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: A double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation 2014;21:291-6. [PMID: ] - PubMed
-
- Khalili M, Eghtesadi S, Mirshafiey A, Eskandari G, Sanoobar M, Sahraian MA, et al. Effect of lipoic acid consumption on oxidative stress among multiple sclerosis patients: A randomized controlled clinical trial. Nutritional Neuroscience 2014;17(1):16-20. [PMID: ] - PubMed
-
- Seifar F, Khalili M, Azimi A. Effect of lipoic acid on oxidative stress in multiple sclerosis patients: A double blind randomised clinical trial [abstract]. European Journal of Neurology 2016;23(S2):825.
Kouchaki 2017 {published data only}
-
- Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clinical Nutrition 2017;36:1245-9. [PMID: ] - PubMed
Mahler 2015 {published data only}
-
- Mähler A, Steiniger J, Bock M, Klug L, Parreidt N, Lorenz M, et al. Green tea and energy metabolism in multiple sclerosis patients [abstract]. Multiple Sclerosis Journal 2013;19(S1):524.
-
- Mähler A, Steiniger J, Bock M, Klug L, Parreidt N, Lorenz M, et al. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: A randomized clinical trial. American Journal of Clinical Nutrition 2015;101(3):487-95. [PMID: ] - PubMed
Malin 2008 {published data only}
-
- Malin S, Cotugna N, Fang C. Effect of creatine supplementation on muscle capacity in individuals with multiple sclerosis. Journal of Dietary Supplements 2008;5(1):20-32. [PMID: ] - PubMed
Markowitz 2009 {published data only}
Millar 1973 {published data only}
Munoz Garcia 2015 {published data only}
-
- Muñoz García D, Midaglia L, Martinez Vilela J, Marín Sánchez M, López González FJ, Arias Gómez M, et al. Associated inosine to interferon: Results of a clinical trial in multiple sclerosis. Acta Neurologica Scandanavia 2015;131:405-10. [PMID: ] - PubMed
Naghashpour 2013 {published data only}
-
- Naghashpour M, Majdinasab N, Shakerinejad G, Kouchak M, Haghighizadeh MH, Jarvandi F, et al. Riboflavin supplementation to patients with multiple sclerosis does not improve disability status nor is riboflavin supplementation correlated to homocysteine. International Journal of Vitamin and Nutrition Research 2013;83(5):281-90. [PMID: ] - PubMed
Orefice 2016 {published data only}
-
- Montella S, Carotenuto A, Orefice NS, Orefice G. A double-blind, randomized, versus-placebo study of palmitoylethanolamide in subjects with relapsing remitting multiple sclerosis: Preliminary results [abstract]. Journal of Neurology 2014;261(S1):S437-8.
-
- Orefice N, Calabrese M, Carotenuto A, Cerillo I, Montella S, Cerullo G, et al. A double-blind, randomized, versus-placebo study of palmitoylethanolamide in relapsing-remitting multiple sclerosis [abstract]. Multiple Sclerosis Journal 2014;20(S1):114-5.
-
- Orefice NS, Alhouayek M, Carotenuto A, Montella S, Barbato F, Comelli A, et al. Oral palmitoylethanolamide treatment is associated with reduced cutaneous adverse effects of interferon-β1a and circulating proinflammatory cytokines in relapsing-remitting multiple sclerosis. Neurotherapeutics 2016;13(2):428-38. [PMID: 26857391 ] - PMC - PubMed
Pantzaris 2013 {published data only}
-
- Pantzaris M, Loukaides G, Ntzani E, Patrikios I. A novel oral medical nutrition formula (PLP10) for the treatment of relapsing-remitting multiple sclerosis: A randomised, double-blind, placebo-controlled proof-of-concept clinical trial [abstract]. Multiple Sclerosis Journal 2012;18(S4):473.
-
- Pantzaris MC, Loukaides GN, Ntzani EE, Patrikios IS. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ Open 2013;3(4):e002170. [PMID: ] - PMC - PubMed
-
- Patrikios I, Loucaides G, Pantzaris M, Crawford M, Ghebremeskel K. Omega-3, omega-6 PUFA and gamma-tocopherol in multiple sclerosis: PLP10 intervention efficacy and red blood cells' membrane lipids composition [abstract]. Multiple Sclerosis Journal 2015;21(S11):323.
-
- Patrikios I, Pantzaris M, Loukaides G, Ntzani E. Oral nutraceutical formula (PLP10) for the treatment of relapsing remitting multiple sclerosis: double-blind, randomized clinical trial [abstract]. Clinical Nutrition 2012;7(1):266.
Paty 1978 {published data only}
-
- Paty DW, Cousin HK, McDonald LE. Letter: Linoleic acid in multiple sclerosis. Lancet 1975;1(7917):1197-8. [PMID: ] - PubMed
-
- Paty DW, Cousin HK, Read S, Adlakha K. Linoleic acid in multiple sclerosis: Failure to show any therapeutic benefit. Acta Neurologica Scandinavica 1978;58(1):53-8. [PMID: ] - PubMed
-
- Paty DW. Double-blind trial of linoleic acid in multiple sclerosis. Archives of Neurology 1983;40(11):693-4. [PMID: ] - PubMed
Ramirez‐Ramirez 2013 {published data only}
-
- Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG, Pacheco-Moises F, Torres-Sanchez ED, Sorto-Gomez TE, et al. Efficacy of fish oil on serum of TNF α , IL-1 β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxidative Medicine and Cellular Longevity 2013;2013:709493. [PMID: ] - PMC - PubMed
-
- Ramirez-Ramirez VR, Ortiz GG, Islas MA, Sanchez ET, Moises FP, la Rosa AC, et al. Effect of fish oil on cytokines, oxidative stress markers and progression disability in multiple sclerosis [abstract]. Multiple Sclerosis Journal 2012;18(12):1836.
Rezapour‐Firouzi 2013 {published data only}
-
- Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Baradaran B, Sadeghihokmabad E, Mostafaei S, et al. Alteration of delta-6-desaturase (FADS2), secretory phospholipase-A2 (sPLA2) enzymes by Hot-nature diet with co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Complementary Therapies in Medicine 2015;23(5):652-7. [PMID: ] - PubMed
-
- Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Baradaran B, Sadeghihokmabad E, Torbati M, et al. Activity of liver enzymes in multiple sclerosis patients with Hot-nature diet and co-supplemented hemp seed, evening primrose oils intervention. Complementary Therapies in Medicine 2014;22(6):986-93. [PMID: ] - PubMed
-
- Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Farhoudi M, Baradaran B, Ali TM, et al. Erythrocyte membrane fatty acids in multiple sclerosis patients and hot-nature dietary intervention with co-supplemented hemp-seed and evening-primrose oils. African Journal of Traditional, Complementary, and Alternative Medicine 2013;10(6):519-27. [PMID: ] - PMC - PubMed
-
- Rezapour-Firouzi S, Arefhosseini SR, Mehdi F, Mehrangiz E, Baradaran B, Sadeghihokmabad E. Immunomodulatory and therapeutic effects of hot-nature diet and co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Complementary Therapies in Medicine 2013;21(5):473-80. [PMID: ] - PubMed
-
- Rezapour-Firouzi S, Rafie S, Farhoudi M, Ebrahimi-Mamaghani M, Baradaran B, Elyar S, et al. Regulation of lipid-dependent membrane enzymes by hot nature diet with co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Journal of Pure and Applied Microbiology 2013;7(4):2891-901.
Sanoobar 2015 {published data only}
-
- Sanoobar M, Dehghan P, Khalili M, Azimi A, Seifar F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutritional Neuroscience 2016;19(3):138-43. [PMID: ] - PubMed
-
- Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Jazayeri S, Reza Gohari M. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. International Journal of Neuroscience 2013;123(11):776-82. [PMID: ] - PubMed
-
- Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Khodadadi B, Jazayeri S, et al. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: A double blind, placebo, controlled randomized clinical trial. Nutritional Neuroscience 2015;18(4):169-76. [PMID: ] - PubMed
-
- Seifar F, Khalili M, Sanoobar M, Azimi A, Modarresi F. A double blind clinical trial of the effect of coenzyme Q10 on oxidative stress, depression and fatigue in multiple sclerosis patients [abstract]. Multiple Sclerosis Journal 2015;21(S11):330.
Shinto 2016 {published data only}
-
- Shinto L, Marracci G, Stuber L, Bourdette D. Omega-3 fatty acids as an adjunct therapy for depression in multiple sclerosis: A randomized, double-blind placebo-controlled pilot trial [abstract]. Neurology 2010;74(S2):A295.
Tomassini 2004 {published data only}
-
- Tomassini V, Pozzilli C, Onesti E, Pasqualetti P, Marinelli F, Pisani A, et al. Comparison of the effects of acetyl L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: Results of a pilot, randomised, double-blind, crossover trial. Journal of the Neurological Sciences 2004;218(1-2):103-8. [PMID: ] - PubMed
Torkildsen 2012 {published data only}
-
- Kvistad SS, Myhr KM, Holmøy T, Šaltytė Benth J, Wergeland S, Beiske AG, et al. Body mass index influence interferon-beta treatment response in multiple sclerosis. Journal of Neuroimmunology 2015;288:92-7. [PMID: ] - PubMed
-
- Kvistad SS, Myhr KM, Holmøy T, Benth JS, Wergeland S, Løken-Amsrud KI, et al. Body mass index influence disease activity and interferon-beta treatment response in multiple sclerosis [abstract]. Multiple Sclerosis Journal 2015;21(S11):165-6.
-
- Torkildsen O, Bakke S, Beiske A, Bjerve K, Bjornara B, Bjorna I, et al. Omega-3 fatty acids treatment in relapsing-remitting multiple sclerosis [abstract]. European Journal of Neurology 2011;18(S2):49.
-
- Torkildsen O, Beiske A, Hovdal H, Midgard R, Bjorna I, Henriksen O, et al. Effect of omega-3 fatty acid treatment in multiple sclerosis (OFAMS study): Results from a randomised, double-blind, placebo-controlled trial [abstract]. Multiple Sclerosis Journal 2011;17(S1):412.
-
- Torkildsen O, Myhr KM, Beiske A, Bjerve KS, Hovdal H, Midgard R, et al. Alpha-linolenic acid (ALA) serum levels are associated with reduced MRI activity in a prospective cohort of MS patients [abstract]. Multiple Sclerosis Journal 2017;23(S1):15.
Tourbah 2016 {published data only}
-
- Arnold DL, Pelletier J, Berry I, Barillot C, Jean B, Galanaud D, et al. MD1003 in progressive multiple sclerosis: 24-month brain MRI results of the MS-SPI trial [abstract]. Multiple Sclerosis Journal 2017;23(S3):35-6.
-
- Laplaud DA, Gout O, Clavelou P, Pelletier J, Sedel F, Tourbah A. Effect of MD1003 (high-dose biotin) in spinal progressive multiple sclerosis (MS-SPI): Subgroup analyses [abstract]. Multiple Sclerosis Journal 2017;23(S3):402-3.
-
- Lasser R, Bendarraz A, Sedel F. Pharmaceutical-grade high-dose biotin improves outcome in non-active progressive multiple sclerosis: Phase 3 placebo-controlled results [abstract]. Multiple Sclerosis Journal 2017;23(13):NP12.
-
- Papeix C, Lebrun-Frenay C, Defer G, Labauge P, Ruiz M, Simon O, et al. Effect of MD1003 (high-dose biotin) in spinal progressive multiple sclerosis (MS-SPI): EDSS sub-scores [abstract]. Multiple Sclerosis Journal 2017;23(S3):938-9.
-
- Sedel F. High dose biotin for not-active progressive multiple sclerosis [abstract]. Neurotherapeutics 2017;14(3):825.
Weinstock‐Guttman 2005 {published and unpublished data}
-
- Weinstock-Guttman B, Baier M, Lee-Kwen P, Feichter J, Dinehart S, Venkatraman J, et al. A randomized study of low-fat diet with omega-3 fatty acid supplementation in patients with relapsing-remitting multiple sclerosis (RRMS) [abstract]. Neurology 2002;58(7):A461-2.
-
- Weinstock-Guttman B, Baier M, Park Y, Feichter J, Lee-Kwen P, Gallagher E, et al. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2005;73(5):397-404. [PMID: ] - PubMed
Yadav 2005 {published data only}
-
- Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, et al. Lipoic acid in multiple sclerosis: A pilot study. Multiple Sclerosis 2005;11(2):159-65. [CN-00512619] [PMID: ] - PubMed
Yadav 2016 {published data only}
-
- Yadav V, Maracci G, Kim E, Spain R, Cameron M, Overs S, et al. Effects of a very low fat, plant-food based diet on fatigue in multiple sclerosis: Report of a pilot trial [abstract]. Multiple Sclerosis Journal 2014;20(S1):91.
-
- Yadav V, Marracci G, Kim E, Spain R, Cameron M, Overs S, et al. Effects of a low fat plant based diet in multiple sclerosis (MS): results of a 1-year long randomized controlled (RC) study [abstract]. Neurology 2014;82(S10):P6.152.
-
- Yadav V, Marracci G, Kim E, Spain R, Cameron M, Overs S, et al. Low-fat, plant-based diet in multiple sclerosis: A randomized controlled trial. Multiple Sclerosis and Related Disorders 2016;9:80-90. [PMID: ] - PubMed
Zandi‐Esfahan 2017 {published data only}
-
- Zandi-Esfahan S, Fazeli M, Shaygannejad V, Hasheminia J, Badihian S, Aghayerashti M, et al. Evaluating the effect of adding Fish oil to Fingolimod on TNF-alpha, IL1beta, IL6, and IFN-gamma in patients with relapsing-remitting multiple sclerosis: A double-blind randomized placebo-controlled trial. Clinical Neurology and Neurosurgery 2017;163:173-8. [PMID: ] - PubMed
References to studies excluded from this review
Bisaga 2011 {published data only}
-
- Bisaga GN, Odinak MM, Boiko AN, Melnik IuB, Popova NF. Possibilities of treatment of multiple sclerosis exacerbations without corticosteroids: A role of metabolic and antioxidant therapy. Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova 2011;111(2):44-8. [PMID: ] - PubMed
Bisaga 2012 {published data only}
-
- Bisaga GN, Odinak MM, Boiko AN, Melnik YB, Popova NF. Treatment of exacerbations of multiple sclerosis without the use of corticosteroids: the role of metabolic and antioxidant therapy. Neuroscience and Behavioral Physiology 2012;42(2):123-7.
Bitarafan 2013 {published data only}
-
- Bitarafan S, Harirchian MH, Sahraian MA, Keramatipour M, Beladi Moghadam N, Togha M, et al. Impact of vitamin A supplementation on RAR gene expression in multiple sclerosis patients. Journal of Molecular Neuroscience 2013;51(2):478-84. [PMID: ] - PubMed
Bittner 2016 {published data only}
-
- Bittner F, Murchison C, Bourdette D, Spain R. The pharmacokinetics of lipoic acid at baseline and 48 weeks in secondary progressive multiple sclerosis patients. Annals of Neurology 2016;80(S20):S117.
Cendrowski 1982 {published data only}
-
- Cendrowski W. Unsaturated fatty acids in the immunology and therapy of multiple sclerosis. Polski Tygodnik Lekarski 1982;37(8):225-7. - PubMed
Cignarella 2017 {published data only}
-
- Cignarella F, Cantoni C, Ghezzi L, Zhou Y, Cross AH, Piccio L. Intermittent fasting in experimental autoimmune encephalomyelitis and multiple sclerosis. Multiple Sclerosis Journal 2017;23(S1):69.
Coe 2017 {published data only}
-
- Coe S, Axelsson E, Murphy V, Collett J, Clegg M, Izadi H, et al. The effect of high flavonoid cocoa on fatigue in people with multiple sclerosis. Multiple Sclerosis Journal 2016;22(S3):823. - PubMed
-
- Coe S, Axelsson E, Murphy V, Santos M, Collett J, Clegg M, et al. Flavonoid rich dark cocoa may improve fatigue in people with multiple sclerosis, yet has no effect on glycaemic response: An exploratory trial. Clinical Nutrition ESPEN 2017;21:20-5. [PMID: 30014865 ] - PubMed
Dworkin 1981 {published data only}
-
- Dworkin RH. Linoleic acid and multiple sclerosis. Lancet 1981;1(8230):1153-4. [PMID: ] - PubMed
Dworkin 1984 {published data only}
-
- Dworkin RH, Bates D, Millar JH, Paty DW. Linoleic acid and multiple sclerosis: A reanalysis of three double-blind trials. Neurology 1984;34(11):1441-5. [PMID: ] - PubMed
Eghtesadi 2015 {published data only}
-
- Eghtesadi S, Khalili M, Azimi A, Mirshafiey A, Sahraian M, Motevalian A, et al. Study of the effect of lipoic acid consumption on cytokine profile: A double-blind randomized clinical trial. Annals of Nutrition and Metabolism 2015;67(S1):301.
Field 1979 {published data only}
Fitzgerald 2017 {published data only}
-
- Fitzgerald K, Vizthum D, Henry-Barron B, Baer D, Sullivan P, Cassard S, et al. Calorie restriction diets and changes in the metabolome in people with multiple sclerosis. Multiple Sclerosis Journal 2017;23(S3):664-5.
-
- Fitzgerald K, Vizthum D, Henry-Barron B, Baer D, Sullivan P, Cassard S, et al. Concomitant changes in weight and in sleep quality among people with multiple sclerosis. Multiple Sclerosis Journal 2017;23(S3):761.
-
- Fitzgerald KC, Vizthum D, Henry-Barron B, Cassard S, Sullivan P, Baer D, et al. Effects of intermittent calorie restriction on weight, fat mass, lean mass, visceral adipose tissue: Results from a pilot controlled-feeding study in multiple sclerosis patients. Neurology 2017;88(S16):P3.390.
-
- Fitzgerald SK, Vizthum D, Barron B, Sullivan P, Baer D, Mowry EM. Calorie restriction diets and changes in the metabolome in people with multiple. Annals of Neurology 2017;82(S21):S191.
Gasperini 2011 {published data only}
-
- Gasperini C, Sormani MP, Galgani S, Stromillo ML, Scagnolari C, Solaro C, et al. Evaluation of efficacy of an add-on therapy with cianocobalamine (Vitamin B12) plus calcium levofolinate in relapsing-remitting multiple sclerosis patients already in treatment with interferon beta over a period of 24 months for a better longterm outcome (ADVANCE). Multiple Sclerosis Journal 2011;17:S208-9.
Harbige 2007 {published data only}
-
- Harbige LS, Sharief MK. Polyunsaturated fatty acids in the pathogenesis and treatment of multiple sclerosis. British Journal of Nutrition 2007;98(S1):S46-S53. [PMID: ] - PubMed
Holmoy 2013 {published data only}
Jafarirad 2012 {published data only}
-
- Jafarirad S, Siassi F, Harirchian MH, Sahraian MA, Eshraghian MR, Shokri F, et al. The effect of vitamin A supplementation on stimulated T-cell proliferation with myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Journal of Neurosciences in Rural Practice 2012;3(3):294-8. [PMID: ] - PMC - PubMed
Jafarirad 2013 {published data only}
Kouchaki 2018 {published data only}
-
- Kouchaki E, Afarini M, Abolhassani J, Mirhosseini N, Bahmani F, Masoud SA, et al. High-dose ω-3 fatty acid plus vitamin D3 supplementation affects clinical symptoms and metabolic status of patients with multiple sclerosis: a randomized controlled clinical trial. Journal of Nutrition 2018;148(8):1380-6. - PubMed
Lambert 2003 {published data only}
-
- Lambert CP, Archer RL, Carrithers JA, Fink WJ, Evans WJ, Trappe TA. Influence of creatine monohydrate ingestion on muscle metabolites and intense exercise capacity in individuals with multiple sclerosis. Archives of Physical Medicine and Rehabilitation 2003;84(8):1206-10. [PMID: ] - PubMed
Lieben 2017 {published data only}
-
- Lieben CK, Blokland A, Deutz NE, Jansen W, Han G, Hupperts RM. Intake of tryptophan-enriched whey protein acutely enhances recall of positive loaded words in patients with multiple sclerosis. Clinical Nutrition 2018;37(1):321-8. [PMID: ] - PubMed
Loder 2002 {published data only}
-
- Loder C, Allawi J, Horrobin D. Treatment of multiple sclerosis with lofepramine, L-phenylalanine and vitamin B12: Mechanism of action and clinical importance: roles of the locus coeruleus and central noradrenergic systems. Medical Hypotheses 2002;59(5):594-602. [PMID: ] - PubMed
Lopes De Carvalho 2012 {published data only}
-
- Lopes De Carvalho L, Francavilla G, Motta R, Brichetto G. D-mannose, cranberry and Vitamin C are effective in preventing urinary tract infections in multiple sclerosis subjects. Multiple Sclerosis Journal 2012;18(5):S12-S13.
Lovera 2015 {published data only}
-
- Lovera J, Ramos A, Devier D, Garrison V, Kovner B, Reza T, et al. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: Phase I single group and phase II randomized placebo-controlled studies. Journal of Neurological Sciences 2015;358(1-2):46-52. [PMID: ] - PMC - PubMed
Mauriz 2013 {published data only}
-
- Mauriz E, Laliena A, Vallejo D, Tuñón M, Rodriguez-López J, Rodriguez-Pérez R, et al. Effects of a low-fat diet with antioxidant supplementation on biochemical markers of multiple sclerosis long-term care residents. Nutricion Hospitalaria 2013;28(6):2229-35. [PMID: ] - PubMed
Mauriz 2014 {published data only}
-
- Mauriz E, Vallejo D, Tuñón M, Rodriguez-López J, Rodriguez-Pérez R, Sanz-Gómez J, et al. Effects of dietary supplementation with lemon verbena extracts on serum inflammatory markers of multiple sclerosis patients. Nutricion Hospitalaria 2014;31(2):764-71. [PMID: ] - PubMed
Mertin 1973 {published data only}
Meyer‐Rienecker 1976 {published data only}
-
- Meyer-Rienecker JH, Jenssen HL, Kohler H, Field EJ, Shenton BK. Effect of gamma-linolenate in multiple sclerosis. Lancet 1976;2(7992):966. - PubMed
Millar 1984 {published data only}
-
- Millar H. Preliminary-results of double-blind linoleic-acid treatment trials in multiple-sclerosis carried out in Belfast, Newcastle-Upon-Tyne and London, Ontario. Irish Journal of Medical Science 1984;153(4):153.
Moccia 2019 {published data only}
Mohammadzadeh Honarvar 2013 {published data only}
-
- Mohammadzadeh Honarvar N, Harirchian M, Koohdani F, Siassi F, Abdolahi M, Bitarafan S, et al. The effect of vitamin A supplementation on retinoic acid-related orphan receptor γt (RORγt) and interleukin-17 (IL-17) gene expression in Avonex-treated multiple sclerotic patients. Journal of Molecular Neuroscience 2013;51(3):749-53. [PMID: ] - PubMed
Mohammadzadeh Honarvar 2016 {published data only}
-
- Mohammadzadeh Honarvar N, Harirchian MH, Abdolahi M, Abedi E, Bitarafan S, Koohdani F, et al. Retinyl palmitate supplementation modulates T-bet and interferon gamma gene expression in multiple sclerosis patients. Journal of Molecular Neuroscience 2016;59(3):360-5. [PMID: 27122150] - PubMed
Saboor‐Yaraghi 2015 {published data only}
-
- Saboor-Yaraghi A, Harirchian M, Mohammadzadeh HN, Bitarafan S, Abdolahi M, Siassi F, et al. The effect of vitamin A supplementation on FoxP3 and TGF-β gene expression in avonex-treated multiple sclerosis patients. Journal of Molecular Neuroscience 2015;56(3):608-12. [PMID: ] - PubMed
Salari 2015 {published data only}
-
- Salari S, Khomand P, Arasteh M, Yousefzamani B, Hassamzadeh K. Zinc sulphate: A reasonable choice for depression management in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Pharmacological Reports 2015;67(3):606-9. [PMID: ] - PubMed
Saresella 2017 {published data only}
Schultz 1984 {published data only}
-
- Schultz A. Efficacy of cranberry juice and ascorbic acid in acidifying the urine in multiple sclerosis subjects. Journal of Community Health Nursing 1984;1(3):159-69. [PMID: ] - PubMed
Shinto 2008 {published data only}
Simpson 1985 {published data only}
-
- Simpson L, Shand B, Olds R. Dietary supplementation with Efamol and multiple sclerosis. New Zealand Medical Journal 1985;98:1053. - PubMed
Skakonik 1963 {published data only}
-
- Skakonik W, Eisner M. Trials in the treatment of multiple sclerosis with diabetol associated with diets poor in carbohydrates. Annales Academiae Medicae Stetinensis 1963;9:267-70. - PubMed
Spitsin 2010 {published data only}
-
- Spitsin S, Markowitz C, Zimmerman V, Koprowski H, Hooper D. Modulation of serum uric acid levels by inosine in patients with multiple sclerosis does not affect blood pressure. Journal of Human Hypertension 2010;24(5):359-62. [PMID: ] - PubMed
Swank 1990 {published data only}
-
- Swank RL, Bourdillon RB. Multiple sclerosis: assessment of treatment with a modified low-fat diet. Journal of Nervous and Mental Disease 1960;131:468-88. [PMID: ] - PubMed
-
- Swank RL, Dugan BB. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet 1990;336(8706):37-9. [PMID: ] - PubMed
-
- Swank RL, Goodwin J. Review of MS patient survival on a Swank low saturated fat diet. Nutrition 2003;19(2):161-2. [PMID: ] - PubMed
-
- Swank RL, Grimsgaard A. Multiple sclerosis: the lipid relationship. American Journal of Clinical Nutrition 1988;48(6):1387-93. [PMID: ] - PubMed
-
- Swank RL. Multiple sclerosis: Fat-oil relationship. Nutrition 1991;7(5):368-76. [PMID: ] - PubMed
Tamtaji 2017 {published data only}
-
- Tamtaji O, Kouchaki E, Salami M, Aghadavod E, Akbari E, Tajabadi-Ebrahimi M, et al. The effects of probiotic supplementation on gene expression related to inflammation, insulin, and lipids in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Journal of the American College of Nutrition 2017;36(8):660-5. [PMID: 28922099] - PubMed
Toncev 2006 {published data only}
-
- Toncev G. Therapeutic value of serum uric acid levels increasing in the treatment of multiple sclerosis. Vojnosanitetski Pregled 2006;63(10):879-82. [PMID: ] - PubMed
Tran 2018 {published data only}
van Rensburg 2006 {published data only}
-
- Rensburg SJ, Kotze MJ, Hon D, Haug P, Kuyler J, Hendricks M, et al. Iron and the folate-vitamin B12-methylation pathway in multiple sclerosis. Metabolic Brain Disease 2006;21(2-3):121-37. [PMID: ] - PubMed
Wade 2002 {published data only}
-
- Wade DT, Young CA, Chaudhuri KR, Davidson DL. A randomised placebo controlled exploratory study of vitamin B12, lofepramine, and L-phenylalanine (the "Cari Loder regime") in the treatment of multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry 2002;73(3):246-9. [PMID: ] - PMC - PubMed
References to studies awaiting assessment
Bock 2015 {published data only}
-
- Bock M, Michaelsen A, Paul F. Ketogenic diet and prolonged fasting improve health related quality of life and blood lipid profile in multiple sclerosis - A randomized controlled trial. Multiple Sclerosis Journal 2015;21(S11):794-5.
Kanter 2014 {published data only}
-
- Kanter J, Keiner E, Biwer A, Coughlin E, DiFabio B, Salmasinia D, et al. A double-blind placebo controlled study of the effect of beta-alanine, a nonessential amino-acid, on neurologic, motor function, quality of life, and fatigue in patients diagnosed with multiple sclerosis. Neurology 2014;82(10 Suppl 1):P7.253.
Khalili 2017 {published data only}
Loy 2018 {published data only}
Shah 2007 {published data only}
-
- Shah PS, O'Riordan JI, Gold L, Houston G, Donnan P. The role of diet in early relapsing remitting multiple sclerosis - A randomised controlled single-blind pilot study (ongoing clinical trial). European Journal of Neurology 2007;14:287-8.
Tourbah 2018 {published data only}
-
- Tourbah A, Arndt C, Vighetto A, Deburghgraeve V, Pelletier J, Papeix C, et al. Effect of MD1003 (high doses of biotin) in chronic visual loss related to optic neuritis in multiple sclerosis (MS-ON): Results of a pivotal randomized double masked placebo controlled study [abstract]. Neurology 2016;86(S16):S49.005.
-
- Tourbah A, Gout O, Vighetto A, Deburghgraeve V, Pelletier J, Papeix C, et al. MD1003 (high-dose pharmaceutical-grade Biotin) for the treatment of chronic visual loss related to optic neuritis in multiple sclerosis: a randomized, double-blind, placebo-controlled study. CNS Drugs 2018;32(7):661-72. [PMID: ] - PMC - PubMed
References to ongoing studies
NCT01514370 {published data only}
-
- NCT01514370. Dietary supplement of curcumin in subjects with active relapsing multiple sclerosis treated with subcutaneous interferon Beta 1a. ClinicalTrials.gov/show/NCT01514370 (first received 23 January 2012).
NCT01848327 {published data only}
-
- NCT01848327. Caprylic triglyceride for treatment of cognitive impairments in multiple sclerosis. ClinicalTrials.gov/show/NCT01848327 (first received 7 May 2013).
NCT01915433 {published data only}
-
- NCT01915433. Wahls Paleo diet and progressive multiple sclerosis. ClinicalTrials.gov/show/NCT01915433 (first received 2 August 2013).
NCT02664623 {published data only}
-
- NCT02664623. Personalized nutrition advice for optimizing dietary calcium intake in MS patients. ClinicalTrials.gov/show/NCT02664623 (first received 27 January 2016).
NCT02914964 {published data only}
-
- NCT02914964. Dietary approaches to treat multiple sclerosis-related fatigue study. ClinicalTrials.gov/show/NCT02914964 (first received 26 September 2016).
NCT02936037 {published data only}
-
- NCT02936037. Effect of MD1003 in progressive multiple sclerosis (SPI2). ClinicalTrials.gov/show/NCT02936037 (first received 18 October 2016).
NCT02986893 {published data only}
-
- NCT02986893. Pilot diet study for multiple sclerosis. ClinicalTrials.gov/show/NCT02986893 (first received 8 December 2016).
NCT03322982 {published data only}
-
- NCT03322982. Low fat diet for fatigue in MS. ClinicalTrials.gov/show/NCT03322982 (first received 26 October 2017).
NCT03387046 {published data only}
-
- NCT03387046. A pilot study in subjects with relapsing remitting multiple sclerosis (RR-MS). ClinicalTrials.gov/show/NCT03387046 (first received 29 December 2017).
NCT03508414 {published data only}
-
- NCT03508414. Nutritional approaches in multiple sclerosis. ClinicalTrials.gov/show/NCT03508414 (first received 25 April 2018).
Additional references
Adamczyk 2016
AlAmmar 2019
-
- AlAmmar WA, Albeesh FH, Ibrahim LM, Algindan YY, Yamani LZ, Khattab RY. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: a systematic review. Nutritional Neuroscience 2019 Aug 28 [Epub ahead of print]. [PMID: ] - PubMed
Altowaijri 2017
-
- Altowaijri G, Fryman A, Yadav V. Dietary interventions and multiple sclerosis. Current Neurology and Neuroscience Reports 2017;17(3):28. [PMID: ] - PubMed
Atkins 2004
Atlas of MS 2013
-
- Atlas of MS 2013: Mapping multiple sclerosis around the world. www.msif.org/resources (accessed 4 March 2018).
Bagur 2017
Bar‐Or 2010
-
- Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Annals of Neurology 2010;67(4):452-61. [PMID: ] - PubMed
Bjornevik 2017
Boringa 2001
-
- Boringa JB, Lazeron RH, Reuling IE, Adèr HJ, Pfennings L, Lindeboom J, et al. The brief repeatable battery of neuropsychological tests: Normative values allow application in multiple sclerosis clinical practice. Multiple Sclerosis 2001;7(4):263-7. [PMID: ] - PubMed
Bowling 2003
-
- Bowling AC, Stewart TM. Current complementary and alternative therapies for multiple sclerosis. Current Treatment Options in Neurology 2003;5(1):55-68. [PMID: ] - PubMed
Brazier 1992
Claflin 2018
-
- Claflin SB, Mei IA, Taylor BV. Complementary and alternative treatments of multiple sclerosis: A review of the evidence from 2001 to 2016. Journal of Neurology, Neurosurgery, and Psychiatry 2018;89(1):34-41. [PMID: ] - PubMed
Cohen 2012
-
- Cohen JA, Reingold SC, Polman CH, Wolinsky JS. Disability outcome measures in multiple sclerosis clinical trials: Current status and future prospects. Lancet Neurology 2012;11(5):467-76. [PMID: ] - PubMed
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, Version accessed 5 February 2018.Available at covidence.org.
Cree 2016
Dardiotis 2017
-
- Dardiotis E, Arseniou S, Sokratous M, Tsouris Z, Siokas V, Mentis AA, et al. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: A meta-analysis. Multiple Sclerosis and Related Disorders 2017;17:190-7. [PMID: ] - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Egger 1997
Esposito 2018
-
- Esposito S, Bonavita S, Sparaco M, Gallo A, Tedeschi G. The role of diet in multiple sclerosis: A review. Nutritional Neuroscience 2018;21(6):377-90. [PMID: ] - PubMed
Estruch 2013
-
- Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. New England Journal of Medicine 2013;368(14):1279-90. [PMID: ] - PubMed
Fisk 1994
-
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clinical Infectious Diseases 1994;18(S1):S79-S83. [PMID: ] - PubMed
Giovannoni 2018
-
- Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Multiple Sclerosis Journal 2018;24(12):1594-1604. [PMID: ] - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Hauser 2017
-
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. New England Journal of Medicine 2017;376(3):221-34. [PMID: ] - PubMed
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jagannath 2018
Kamm 2014
-
- Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: Current knowledge and future outlook. European Neurology 2014;72(3-4):132-41. [PMID: ] - PubMed
Krupp 1989
-
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46(10):1121-3. [PMID: ] - PubMed
Kurtzke 1983
-
- Kurtzke JF. Rating neurological impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983;33(11):1444-5. [PMID: ] - PubMed
Leong 2009
-
- Leong EM, Semple SJ, Angley M, Siebert W, Petkov J, McKinnon RA. Complementary and alternative medicines and dietary interventions in multiple sclerosis: What is being used in South Australia and why? Complementary Therapies in Medicine 2009;17:216-23. [PMID: ] - PubMed
Lublin 1996
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46(4):907-11. [PMID: ] - PubMed
Lublin 2014
Lucchinetti 2005
-
- Lucchinetti CF, Parisi J, Bruck W. The pathology of multiple sclerosis. Neurologic Clinics 2005;23(1):77-105. [PMID: ] - PubMed
Marrie 2010
McDonald 1977
-
- McDonald WI, Halliday AM. Diagnosis and classification of multiple sclerosis. British Medical Bulletin 1977;33(1):4-9. [PMID: ] - PubMed
McDonald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for MS: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121-7. [PMID: ] - PubMed
Mehta 2009
-
- Mehta LR, Dworkin RH, Schwid SV. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nature Clinical Practice Neurology 2009;5:82-92. [PMID: ] - PubMed
Millar 1967
-
- Millar JH, Vas CJ, Noronha MJ, Liversedge LA, Rawson MD. Long-term treatment of multiple sclerosis with corticotrophin. Lancet 1967;2(7513):429-31. [PMID: ] - PubMed
Montalban 2017
-
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New England Journal of Medicine 2017;376(3):209-20. [PMID: ] - PubMed
Namaka 2008
-
- Namaka M, Crook A, Doupe A, Kler K, Vasconcelos M, Klowak M, et al. Examining the evidence: Complementary adjunctive therapies for multiple sclerosis. Neurological Research 2008;30(7):710-19. [PMID: ] - PubMed
Noseworthy 2000
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New England Journal of Medicine 2000;343(13):938-52. [PMID: ] - PubMed
O'Connor 2012
-
- O’Connor K, Weinstock-Guttman B, Carl E, Kilanowski C, Zivadinov R, Ramanathan M. Patterns of dietary and herbal supplement use by multiple sclerosis patients. Journal of Neurology 2012;259:637-44. [PMID: ] - PubMed
Payne 2001
-
- Payne A. Nutrition and diet in the clinical management of multiple sclerosis. Journal of Human Nutrition and Dietetics 2001;14(5):349-57. [PMID: ] - PubMed
Polman 2005
-
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840-6. [PMID: ] - PubMed
Polman 2011
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Annals of Neurology 1983;13(3):227-31. [PMID: ] - PubMed
Pucci 2004
-
- Pucci E, Cartechini E, Taus C, Giuliani G. Why physicians need to look more closely at the use of complementary and alternative medicine by multiple sclerosis patients. European Journal of Neurology 2004;11:263-7. [PMID: ] - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 2006
-
- Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurology 2006;5(11):949-60. [PMID: ] - PubMed
Schumacher 1965
-
- Schumacher GA, Beede GW, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in multiple sclerosis: Report by the panel of evaluation of experimental trials in multiple sclerosis. Annals of the New York Academy of Sciences 1965;122:552-68. [PMID: ] - PubMed
Schwarz 2005
-
- Schwarz S, Leweling H. Multiple sclerosis and nutrition. Multiple Sclerosis 2005;11(1):24-32. [PMID: ] - PubMed
Swank 2003a
-
- Swank RL, Goodwin J. Review of MS patient survival on a swank low saturated fat diet. Nutrition 2003;19(2):161-2. [PMID: ] - PubMed
Swank 2003b
-
- Swank RL, Goodwin JW. How saturated fats may be a causative factor in multiple sclerosis and other diseases. Nutrition 2003;19(5):478. [PMID: ] - PubMed
Thompson 2018
-
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurology 2018;17(2):162-73. [PMID: ] - PubMed
Tombaugh 2006
-
- Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol 2006;21(1):53-76. [PMID: ] - PubMed
Torkildsen 2016
Trojano 2011
-
- Trojano M, Paolicelli D, Tortorella C, Iaffaldano P, Lucchese G, Di Renzo V, et al. Natural history of multiple sclerosis: Have available therapies impacted long-term prognosis? Neurologic Clinics 2011;29(2):309-21. [PMID: ] - PubMed
Vickrey 1995
-
- Vickrey BG, Hays RD, Harooni R, Myers W, Ellison GW. A health-related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187-206. [PMID: ] - PubMed
Yadav 2010
Yadav 2014
References to other published versions of this review
Farinotti 2003
Farinotti 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

