Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19
- PMID: 32427279
- PMCID: PMC7314133
- DOI: 10.1093/cid/ciaa601
Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19
Abstract
Background: There is no proven antiviral or immunomodulatory therapy for coronavirus disease 2019 (COVID-19). The disease progression associated with the proinflammatory host response prompted us to examine the role of early corticosteroid therapy in patients with moderate to severe COVID-19.
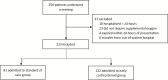
Methods: We conducted a single pretest, single posttest quasi-experiment in a multicenter health system in Michigan from 12 March to 27 March 2020. Adult patients with confirmed moderate to severe COVID were included. A protocol was implemented on 20 March 2020 using early, short-course, methylprednisolone 0.5 to 1 mg/kg/day divided in 2 intravenous doses for 3 days. Outcomes of standard of care (SOC) and early corticosteroid groups were evaluated, with a primary composite endpoint of escalation of care from ward to intensive care unit (ICU), new requirement for mechanical ventilation, and mortality. All patients had at least 14 days of follow-up.
Results: We analyzed 213 eligible subjects, 81 (38%) and 132 (62%) in SOC and early corticosteroid groups, respectively. The composite endpoint occurred at a significantly lower rate in the early corticosteroid group (34.9% vs 54.3%, P = .005). This treatment effect was observed within each individual component of the composite endpoint. Significant reduction in median hospital length of stay was also observed in the early corticosteroid group (5 vs 8 days, P < .001). Multivariate regression analysis demonstrated an independent reduction in the composite endpoint at 14-days controlling for other factors (adjusted odds ratio: 0.41; 95% confidence interval, .22 - .77).
Conclusions: An early short course of methylprednisolone in patients with moderate to severe COVID-19 reduced escalation of care and improved clinical outcomes.
Clinical trials registration: NCT04374071.
Keywords: COVID-19; Corticosteroids; SARS-COV-2; coronavirus; outcomes.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
The Right Time for Steroids in COVID-19.Clin Infect Dis. 2021 Apr 26;72(8):1486-1487. doi: 10.1093/cid/ciaa865. Clin Infect Dis. 2021. PMID: 32585016 Free PMC article. No abstract available.
-
Reply to Fernandez Cruz, et al.Clin Infect Dis. 2021 Apr 26;72(8):1487. doi: 10.1093/cid/ciaa870. Clin Infect Dis. 2021. PMID: 32589700 No abstract available.
Similar articles
-
Comparison of pulse-dose and high-dose corticosteroids with no corticosteroid treatment for COVID-19 pneumonia in the intensive care unit.J Med Virol. 2022 Jan;94(1):349-356. doi: 10.1002/jmv.27351. Epub 2021 Sep 28. J Med Virol. 2022. PMID: 34542192 Free PMC article.
-
Clinical Use of Short-Course and Low-Dose Corticosteroids in Patients With Non-severe COVID-19 During Pneumonia Progression.Front Public Health. 2020 Jul 3;8:355. doi: 10.3389/fpubh.2020.00355. eCollection 2020. Front Public Health. 2020. PMID: 32719766 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Effects of corticosteroids on Covid-19 patients: A systematic review and meta-analysis on clinical outcomes.Pulm Pharmacol Ther. 2022 Feb;72:102107. doi: 10.1016/j.pupt.2021.102107. Epub 2021 Dec 18. Pulm Pharmacol Ther. 2022. PMID: 34933068 Free PMC article. Review.
-
Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2018 Mar 19;3(3):CD006897. doi: 10.1002/14651858.CD006897.pub4. Cochrane Database Syst Rev. 2018. PMID: 29553157 Free PMC article. Review.
Cited by
-
Respiratory viruses: their importance and lessons learned from COVID-19.Eur Respir Rev. 2022 Oct 19;31(166):220051. doi: 10.1183/16000617.0051-2022. Print 2022 Dec 31. Eur Respir Rev. 2022. PMID: 36261158 Free PMC article. Review.
-
Five severe COVID-19 pneumonia patients treated with triple combination therapy with lopinavir/ritonavir, hydroxychloroquine, and interferon β-1b.Int J Antimicrob Agents. 2020 Aug;56(2):106052. doi: 10.1016/j.ijantimicag.2020.106052. Epub 2020 Jun 13. Int J Antimicrob Agents. 2020. PMID: 32544570 Free PMC article. No abstract available.
-
Immunity, endothelial injury and complement-induced coagulopathy in COVID-19.Nat Rev Nephrol. 2021 Jan;17(1):46-64. doi: 10.1038/s41581-020-00357-4. Epub 2020 Oct 19. Nat Rev Nephrol. 2021. PMID: 33077917 Free PMC article. Review.
-
Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: An observational comparative study using routine care data.PLoS One. 2020 Sep 22;15(9):e0239401. doi: 10.1371/journal.pone.0239401. eCollection 2020. PLoS One. 2020. PMID: 32960899 Free PMC article.
-
The roles of methylprednisolone treatment in patients with COVID-19: A systematic review and meta-analysis.Steroids. 2022 Jul;183:109022. doi: 10.1016/j.steroids.2022.109022. Epub 2022 Mar 26. Steroids. 2022. PMID: 35346661 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases and latest updates. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Accessed 7 April 2020
-
- Centers for Disease Control and Prevention. COVID-NET: COVID-19 associated hospitalization surveillance network. Available at: https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html. Accessed 8 April 2020.
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous