Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State
- PMID: 32392282
- PMCID: PMC7215635
- DOI: 10.1001/jama.2020.8630
Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State
Abstract
Importance: Hydroxychloroquine, with or without azithromycin, has been considered as a possible therapeutic agent for patients with coronavirus disease 2019 (COVID-19). However, there are limited data on efficacy and associated adverse events.
Objective: To describe the association between use of hydroxychloroquine, with or without azithromycin, and clinical outcomes among hospital inpatients diagnosed with COVID-19.
Design, setting, and participants: Retrospective multicenter cohort study of patients from a random sample of all admitted patients with laboratory-confirmed COVID-19 in 25 hospitals, representing 88.2% of patients with COVID-19 in the New York metropolitan region. Eligible patients were admitted for at least 24 hours between March 15 and 28, 2020. Medications, preexisting conditions, clinical measures on admission, outcomes, and adverse events were abstracted from medical records. The date of final follow-up was April 24, 2020.
Exposures: Receipt of both hydroxychloroquine and azithromycin, hydroxychloroquine alone, azithromycin alone, or neither.
Main outcomes and measures: Primary outcome was in-hospital mortality. Secondary outcomes were cardiac arrest and abnormal electrocardiogram findings (arrhythmia or QT prolongation).
Results: Among 1438 hospitalized patients with a diagnosis of COVID-19 (858 [59.7%] male, median age, 63 years), those receiving hydroxychloroquine, azithromycin, or both were more likely than those not receiving either drug to have diabetes, respiratory rate >22/min, abnormal chest imaging findings, O2 saturation lower than 90%, and aspartate aminotransferase greater than 40 U/L. Overall in-hospital mortality was 20.3% (95% CI, 18.2%-22.4%). The probability of death for patients receiving hydroxychloroquine + azithromycin was 189/735 (25.7% [95% CI, 22.3%-28.9%]), hydroxychloroquine alone, 54/271 (19.9% [95% CI, 15.2%-24.7%]), azithromycin alone, 21/211 (10.0% [95% CI, 5.9%-14.0%]), and neither drug, 28/221 (12.7% [95% CI, 8.3%-17.1%]). In adjusted Cox proportional hazards models, compared with patients receiving neither drug, there were no significant differences in mortality for patients receiving hydroxychloroquine + azithromycin (HR, 1.35 [95% CI, 0.76-2.40]), hydroxychloroquine alone (HR, 1.08 [95% CI, 0.63-1.85]), or azithromycin alone (HR, 0.56 [95% CI, 0.26-1.21]). In logistic models, compared with patients receiving neither drug cardiac arrest was significantly more likely in patients receiving hydroxychloroquine + azithromycin (adjusted OR, 2.13 [95% CI, 1.12-4.05]), but not hydroxychloroquine alone (adjusted OR, 1.91 [95% CI, 0.96-3.81]) or azithromycin alone (adjusted OR, 0.64 [95% CI, 0.27-1.56]), . In adjusted logistic regression models, there were no significant differences in the relative likelihood of abnormal electrocardiogram findings.
Conclusions and relevance: Among patients hospitalized in metropolitan New York with COVID-19, treatment with hydroxychloroquine, azithromycin, or both, compared with neither treatment, was not significantly associated with differences in in-hospital mortality. However, the interpretation of these findings may be limited by the observational design.
Conflict of interest statement
Figures
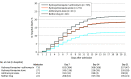
Comment in
-
Impact of Hydroxychloroquine on Antibody Responses to the SARS-CoV-2 Coronavirus.Front Immunol. 2020 Aug 4;11:1739. doi: 10.3389/fimmu.2020.01739. eCollection 2020. Front Immunol. 2020. PMID: 32849619 Free PMC article. No abstract available.
-
Dangers of the use of hydroxychloroquine and azithromycin combination in COVID-19 patients.Travel Med Infect Dis. 2020 Nov-Dec;38:101881. doi: 10.1016/j.tmaid.2020.101881. Epub 2020 Sep 18. Travel Med Infect Dis. 2020. PMID: 32956851 Free PMC article. No abstract available.
Similar articles
-
Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19.Int J Infect Dis. 2020 Aug;97:396-403. doi: 10.1016/j.ijid.2020.06.099. Epub 2020 Jul 2. Int J Infect Dis. 2020. PMID: 32623082 Free PMC article.
-
Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19).JAMA Cardiol. 2020 Sep 1;5(9):1036-1041. doi: 10.1001/jamacardio.2020.1834. JAMA Cardiol. 2020. PMID: 32936252 Free PMC article.
-
Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study.PLoS One. 2020 Aug 13;15(8):e0237693. doi: 10.1371/journal.pone.0237693. eCollection 2020. PLoS One. 2020. PMID: 32790733 Free PMC article.
-
Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection.CMAJ. 2020 Apr 27;192(17):E450-E453. doi: 10.1503/cmaj.200528. Epub 2020 Apr 8. CMAJ. 2020. PMID: 32269021 Free PMC article. Review. No abstract available.
-
Will Hydroxychloroquine Still Be a Game-Changer for COVID-19 by Combining Azithromycin?Front Immunol. 2020 Aug 7;11:1969. doi: 10.3389/fimmu.2020.01969. eCollection 2020. Front Immunol. 2020. PMID: 32849658 Free PMC article. Review.
Cited by
-
Modalities and Mechanisms of Treatment for Coronavirus Disease 2019.Front Pharmacol. 2021 Feb 8;11:583914. doi: 10.3389/fphar.2020.583914. eCollection 2020. Front Pharmacol. 2021. PMID: 33643033 Free PMC article. Review.
-
Pharmacokinetics and Pharmacological Properties of Chloroquine and Hydroxychloroquine in the Context of COVID-19 Infection.Clin Pharmacol Ther. 2020 Dec;108(6):1135-1149. doi: 10.1002/cpt.1993. Epub 2020 Sep 1. Clin Pharmacol Ther. 2020. PMID: 32687630 Free PMC article. Review.
-
A Review on Current Repurposing Drugs for the Treatment of COVID-19: Reality and Challenges.SN Compr Clin Med. 2020;2(10):1777-1789. doi: 10.1007/s42399-020-00485-9. Epub 2020 Aug 31. SN Compr Clin Med. 2020. PMID: 32904710 Free PMC article. Review.
-
The COVID-19 pandemic: A community approach.Clin Transplant. 2020 Nov;34(11):e14059. doi: 10.1111/ctr.14059. Epub 2020 Sep 28. Clin Transplant. 2020. PMID: 32762055 Free PMC article. Review.
-
Quinine Inhibits Infection of Human Cell Lines with SARS-CoV-2.Viruses. 2021 Apr 9;13(4):647. doi: 10.3390/v13040647. Viruses. 2021. PMID: 33918670 Free PMC article.
References
-
- The Center for Systems Science and Engineering at Johns Hopkins University COVID-19 dashboard. 2020. Accessed March 24, 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594...67b48e9ecf6
-
- New York State Department of Health COVID-19 Tracker. 2020. Accessed May 7, 2020. https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOV...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

