Alzheimer's Disease-Like Neurodegeneration in Porphyromonas gingivalis Infected Neurons with Persistent Expression of Active Gingipains
- PMID: 32390638
- PMCID: PMC7369049
- DOI: 10.3233/JAD-200393
Alzheimer's Disease-Like Neurodegeneration in Porphyromonas gingivalis Infected Neurons with Persistent Expression of Active Gingipains
Abstract
Background: Porphyromonas gingivalis (P. gingivalis) and its gingipain virulence factors have been identified as pathogenic effectors in Alzheimer's disease (AD). In a recent study we demonstrated the presence of gingipains in over 90% of postmortem AD brains, with gingipains localizing to the cytoplasm of neurons. However, infection of neurons by P. gingivalis has not been previously reported.
Objective: To demonstrate intraneuronal P. gingivalis and gingipain expression in vitro after infecting neurons derived from human inducible pluripotent stem cells (iPSC) with P. gingivalis for 24, 48, and 72 h.
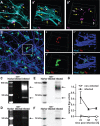
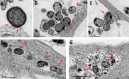
Methods: Infection was characterized by transmission electron microscopy, confocal microscopy, and bacterial colony forming unit assays. Gingipain expression was monitored by immunofluorescence and RT-qPCR, and protease activity monitored with activity-based probes. Neurodegenerative endpoints were assessed by immunofluorescence, western blot, and ELISA.
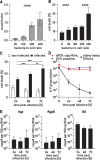
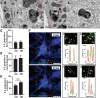
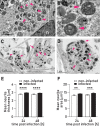
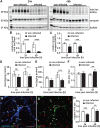
Results: Neurons survived the initial infection and showed time dependent, infection induced cell death. P. gingivalis was found free in the cytoplasm or in lysosomes. Infected neurons displayed an accumulation of autophagic vacuoles and multivesicular bodies. Tau protein was strongly degraded, and phosphorylation increased at T231. Over time, the density of presynaptic boutons was decreased.
Conclusion: P. gingivalis can invade and persist in mature neurons. Infected neurons display signs of AD-like neuropathology including the accumulation of autophagic vacuoles and multivesicular bodies, cytoskeleton disruption, an increase in phospho-tau/tau ratio, and synapse loss. Infection of iPSC-derived mature neurons by P. gingivalis provides a novel model system to study the cellular mechanisms leading to AD and to investigate the potential of new therapeutic approaches.
Keywords: Alzheimer’s disease; Porphyromonas gingivalis; gingipain cysteine endopeptidases; in vitro techniques; multivesicular bodies; synapses; tau protein.
Conflict of interest statement
Authors’ disclosures are available online (
Figures
Similar articles
-
Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors.Sci Adv. 2019 Jan 23;5(1):eaau3333. doi: 10.1126/sciadv.aau3333. eCollection 2019 Jan. Sci Adv. 2019. PMID: 30746447 Free PMC article.
-
Microglial Cathepsin B and Porphyromonas gingivalis Gingipains as Potential Therapeutic Targets for Sporadic Alzheimer's Disease.CNS Neurol Disord Drug Targets. 2020;19(7):495-502. doi: 10.2174/1871527319666200708125130. CNS Neurol Disord Drug Targets. 2020. PMID: 32640970 Review.
-
Secreted gingipains from Porphyromonas gingivalis induce microglia migration through endosomal signaling by protease-activated receptor 2.Neurochem Int. 2020 Nov;140:104840. doi: 10.1016/j.neuint.2020.104840. Epub 2020 Aug 25. Neurochem Int. 2020. PMID: 32858090
-
Porphyromonas gingivalis and Alzheimer disease: Recent findings and potential therapies.J Periodontol. 2020 Oct;91 Suppl 1(Suppl 1):S45-S49. doi: 10.1002/JPER.20-0104. Epub 2020 Aug 6. J Periodontol. 2020. PMID: 32533852 Free PMC article.
-
Role of gingipains R in the pathogenesis of Porphyromonas gingivalis-mediated periodontal disease.Clin Infect Dis. 1999 Mar;28(3):456-65. doi: 10.1086/515156. Clin Infect Dis. 1999. PMID: 10194062 Review.
Cited by
-
Porphyromonas gingivalis Induces Proinflammatory Cytokine Expression Leading to Apoptotic Death through the Oxidative Stress/NF-κB Pathway in Brain Endothelial Cells.Cells. 2021 Nov 5;10(11):3033. doi: 10.3390/cells10113033. Cells. 2021. PMID: 34831265 Free PMC article.
-
A review of the roles of pathogens in Alzheimer's disease.Front Neurosci. 2024 Aug 19;18:1439055. doi: 10.3389/fnins.2024.1439055. eCollection 2024. Front Neurosci. 2024. PMID: 39224577 Free PMC article. Review.
-
Recent advances on drug development and emerging therapeutic agents for Alzheimer's disease.Mol Biol Rep. 2021 Jul;48(7):5629-5645. doi: 10.1007/s11033-021-06512-9. Epub 2021 Jun 28. Mol Biol Rep. 2021. PMID: 34181171 Free PMC article. Review.
-
Characterization of Human Genes Modulated by Porphyromonas gingivalis Highlights the Ribosome, Hypothalamus, and Cholinergic Neurons.Front Immunol. 2021 Jun 14;12:646259. doi: 10.3389/fimmu.2021.646259. eCollection 2021. Front Immunol. 2021. PMID: 34194426 Free PMC article.
-
Human Herpesviruses 6A and 6B in Brain Diseases: Association versus Causation.Clin Microbiol Rev. 2020 Nov 11;34(1):e00143-20. doi: 10.1128/CMR.00143-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33177186 Free PMC article. Review.
References
-
- Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J (2019) Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5, eaau3333. - PMC - PubMed
-
- Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, Aljewari HW, O’Brien-Simpson NM, Reynolds EC, Watanabe K (2018) Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One 13, e0204941. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

