Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury
- PMID: 32375169
- PMCID: PMC7850069
- DOI: 10.1093/brain/awaa116
Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury
Abstract
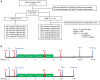
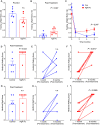
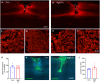
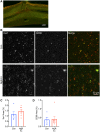
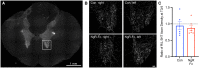
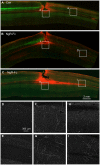
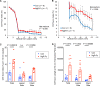
After CNS trauma such as spinal cord injury, the ability of surviving neural elements to sprout axons, reorganize neural networks and support recovery of function is severely restricted, contributing to chronic neurological deficits. Among limitations on neural recovery are myelin-associated inhibitors functioning as ligands for neuronal Nogo receptor 1 (NgR1). A soluble decoy (NgR1-Fc, AXER-204) blocks these ligands and provides a means to promote recovery of function in multiple preclinical rodent models of spinal cord injury. However, the safety and efficacy of this reagent in non-human primate spinal cord injury and its toxicological profile have not been described. Here, we provide evidence that chronic intrathecal and intravenous administration of NgR1-Fc to cynomolgus monkey and to rat are without evident toxicity at doses of 20 mg and greater every other day (≥2.0 mg/kg/day), and far greater than the projected human dose. Adult female African green monkeys underwent right C5/6 lateral hemisection with evidence of persistent disuse of the right forelimb during feeding and right hindlimb during locomotion. At 1 month post-injury, the animals were randomized to treatment with vehicle (n = 6) or 0.10-0.17 mg/kg/day of NgR1-Fc (n = 8) delivered via intrathecal lumbar catheter and osmotic minipump for 4 months. One animal was removed from the study because of surgical complications of the catheter, but no treatment-related adverse events were noted in either group. Animal behaviour was evaluated at 6-7 months post-injury, i.e. 1-2 months after treatment cessation. The use of the impaired forelimb during spontaneous feeding and the impaired hindlimb during locomotion were both significantly greater in the treatment group. Tissue collected at 7-12 months post-injury showed no significant differences in lesion size, fibrotic scar, gliosis or neuroinflammation between groups. Serotoninergic raphespinal fibres below the lesion showed no deficit, with equal density on the lesioned and intact side below the level of the injury in both groups. Corticospinal axons traced from biotin-dextran-amine injections in the left motor cortex were equally labelled across groups and reduced caudal to the injury. The NgR1-Fc group tissue exhibited a significant 2-3-fold increased corticospinal axon density in the cervical cord below the level of the injury relative to the vehicle group. The data show that NgR1-Fc does not have preclinical toxicological issues in healthy animals or safety concerns in spinal cord injury animals. Thus, it presents as a potential therapeutic for spinal cord injury with evidence for behavioural improvement and growth of injured pathways in non-human primate spinal cord injury.
Keywords: Nogo receptor; axon; oligodendrocyte; regeneration; spinal cord injury.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Inhibiting an inhibitor: a decoy to recover dexterity after spinal cord injury.Brain. 2020 Jun 1;143(6):1618-1622. doi: 10.1093/brain/awaa175. Brain. 2020. PMID: 32543695 Free PMC article.
Similar articles
-
Comprehensive Corticospinal Labeling with mu-crystallin Transgene Reveals Axon Regeneration after Spinal Cord Trauma in ngr1-/- Mice.J Neurosci. 2015 Nov 18;35(46):15403-18. doi: 10.1523/JNEUROSCI.3165-15.2015. J Neurosci. 2015. PMID: 26586827 Free PMC article.
-
Human NgR-Fc decoy protein via lumbar intrathecal bolus administration enhances recovery from rat spinal cord contusion.J Neurotrauma. 2014 Dec 15;31(24):1955-66. doi: 10.1089/neu.2014.3355. Epub 2014 Oct 16. J Neurotrauma. 2014. PMID: 24964223 Free PMC article.
-
Recovery from chronic spinal cord contusion after Nogo receptor intervention.Ann Neurol. 2011 Nov;70(5):805-21. doi: 10.1002/ana.22527. Ann Neurol. 2011. PMID: 22162062 Free PMC article.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
-
Mechanisms of CNS myelin inhibition: evidence for distinct and neuronal cell type specific receptor systems.Restor Neurol Neurosci. 2008;26(2-3):97-115. Restor Neurol Neurosci. 2008. PMID: 18820405 Free PMC article. Review.
Cited by
-
Inhibiting an inhibitor: a decoy to recover dexterity after spinal cord injury.Brain. 2020 Jun 1;143(6):1618-1622. doi: 10.1093/brain/awaa175. Brain. 2020. PMID: 32543695 Free PMC article.
-
Molecular approaches for spinal cord injury treatment.Neural Regen Res. 2023 Jan;18(1):23-30. doi: 10.4103/1673-5374.344830. Neural Regen Res. 2023. PMID: 35799504 Free PMC article. Review.
-
Advances in spinal cord injury: insights from non-human primates.Neural Regen Res. 2024 Nov 1;19(11):2354-2364. doi: 10.4103/NRR.NRR-D-23-01505. Epub 2024 Jan 31. Neural Regen Res. 2024. PMID: 38526271 Free PMC article.
-
Lateral olfactory tract usher substance (LOTUS), an endogenous Nogo receptor antagonist, ameliorates disease progression in amyotrophic lateral sclerosis model mice.Cell Death Discov. 2023 Dec 14;9(1):454. doi: 10.1038/s41420-023-01758-7. Cell Death Discov. 2023. PMID: 38097540 Free PMC article.
-
When Spinal Neuromodulation Meets Sensorimotor Rehabilitation: Lessons Learned From Animal Models to Regain Manual Dexterity After a Spinal Cord Injury.Front Rehabil Sci. 2021 Dec 7;2:755963. doi: 10.3389/fresc.2021.755963. eCollection 2021. Front Rehabil Sci. 2021. PMID: 36188826 Free PMC article. Review.
References
-
- Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al.Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 2018; 379: 1244–50. - PubMed
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, et al.PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 2008; 322: 967–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

