Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2
- PMID: 32333836
- PMCID: PMC7181998
- DOI: 10.1016/j.cell.2020.04.004
Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2
Abstract
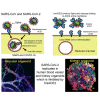
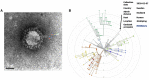
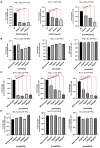
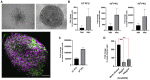
We have previously provided the first genetic evidence that angiotensin converting enzyme 2 (ACE2) is the critical receptor for severe acute respiratory syndrome coronavirus (SARS-CoV), and ACE2 protects the lung from injury, providing a molecular explanation for the severe lung failure and death due to SARS-CoV infections. ACE2 has now also been identified as a key receptor for SARS-CoV-2 infections, and it has been proposed that inhibiting this interaction might be used in treating patients with COVID-19. However, it is not known whether human recombinant soluble ACE2 (hrsACE2) blocks growth of SARS-CoV-2. Here, we show that clinical grade hrsACE2 reduced SARS-CoV-2 recovery from Vero cells by a factor of 1,000-5,000. An equivalent mouse rsACE2 had no effect. We also show that SARS-CoV-2 can directly infect engineered human blood vessel organoids and human kidney organoids, which can be inhibited by hrsACE2. These data demonstrate that hrsACE2 can significantly block early stages of SARS-CoV-2 infections.
Keywords: COVID-19; angiotensin converting enzyme 2; blood vessels; human organoids; kidney; severe acute respiratory syndrome coronavirus; spike glycoproteins; treatment.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.M.P. declares a conflict of interest as a founder, supervisory board member, and shareholder of Apeiron Biologics. G.W. is an employee of Apeiron Biologics. Apeiron holds a patent on the use of ACE2 for the treatment of lung, heart, or kidney injury and applied for a patent to treat COVID-19 with hrsACE2 and use organoids to test new drugs for SARS-CoV-2 infections. R.C. and M.S. are employees of STEMCELL Technologies Inc. A.S.S. has been a consultant to Apeiron Biologics. All other authors declare no competing interests.
Figures
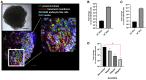
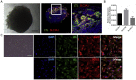

Comment in
-
Blockade of SARS-CoV-2 infection by recombinant soluble ACE2.Kidney Int. 2020 Jun;97(6):1091-1093. doi: 10.1016/j.kint.2020.04.009. Epub 2020 Apr 14. Kidney Int. 2020. PMID: 32354636 Free PMC article. No abstract available.
Similar articles
-
Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19.Mol Biol Rep. 2020 Jun;47(6):4383-4392. doi: 10.1007/s11033-020-05478-4. Epub 2020 May 14. Mol Biol Rep. 2020. PMID: 32410141 Free PMC article.
-
Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19.J Med Virol. 2020 Jul;92(7):726-730. doi: 10.1002/jmv.25785. Epub 2020 Apr 5. J Med Virol. 2020. PMID: 32221983 Free PMC article. Review.
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19.Rev Med Virol. 2020 Sep;30(5):e2119. doi: 10.1002/rmv.2119. Epub 2020 Jun 25. Rev Med Virol. 2020. PMID: 32584474 Free PMC article. Review.
-
Could a specific ACE2 activator drug improve the clinical outcome of SARS-CoV-2? A potential pharmacological insight.Expert Rev Clin Pharmacol. 2020 Aug;13(8):807-811. doi: 10.1080/17512433.2020.1798760. Epub 2020 Jul 25. Expert Rev Clin Pharmacol. 2020. PMID: 32686527 Free PMC article. No abstract available.
Cited by
-
Unveiling the Interplay-Vitamin D and ACE-2 Molecular Interactions in Mitigating Complications and Deaths from SARS-CoV-2.Biology (Basel). 2024 Oct 16;13(10):831. doi: 10.3390/biology13100831. Biology (Basel). 2024. PMID: 39452140 Free PMC article. Review.
-
Therapeutic Strategies Against COVID-19 and Structural Characterization of SARS-CoV-2: A Review.Front Microbiol. 2020 Jul 14;11:1723. doi: 10.3389/fmicb.2020.01723. eCollection 2020. Front Microbiol. 2020. PMID: 32765482 Free PMC article. Review.
-
Targeting the SARS-CoV-2-spike protein: from antibodies to miniproteins and peptides.RSC Med Chem. 2020 Dec 17;12(2):197-202. doi: 10.1039/d0md00385a. RSC Med Chem. 2020. PMID: 34041482 Free PMC article. Review.
-
Endotheliitis and Endothelial Dysfunction in Patients with COVID-19: Its Role in Thrombosis and Adverse Outcomes.J Clin Med. 2020 Jun 15;9(6):1862. doi: 10.3390/jcm9061862. J Clin Med. 2020. PMID: 32549229 Free PMC article.
-
Coronavirus disease 2019 in chronic kidney disease.Clin Kidney J. 2020 Jul 16;13(3):297-306. doi: 10.1093/ckj/sfaa104. eCollection 2020 Jun. Clin Kidney J. 2020. PMID: 32699615 Free PMC article. Review.
References
-
- Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., Lucas J.M., Nelson P.S., Pöhlmann S., Soilleux E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE. 2012;7:e35876. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

