Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma
- PMID: 32327728
- PMCID: PMC7581629
- DOI: 10.1038/s41375-020-0828-7
Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma
Abstract
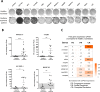
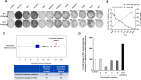
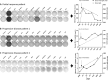
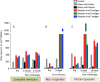
Oncolytic virus therapy leads to immunogenic death of virus-infected tumor cells and this has been shown in preclinical models to enhance the cytotoxic T-lymphocyte response against tumor-associated antigens (TAAs), leading to killing of uninfected tumor cells. To investigate whether oncolytic virotherapy can increase immune responses to tumor antigens in human subjects, we studied T-cell responses against a panel of known myeloma TAAs using PBMC samples obtained from ten myeloma patients before and after systemic administration of an oncolytic measles virus encoding sodium iodide symporter (MV-NIS). Despite their prior exposures to multiple immunosuppressive antimyeloma treatment regimens, T-cell responses to some of the TAAs were detectable even before measles virotherapy. Measurable baseline T-cell responses against MAGE-C1 and hTERT were present. Furthermore, MV-NIS treatment significantly (P < 0.05) increased T-cell responses against MAGE-C1 and MAGE-A3. Interestingly, one patient who achieved complete remission after MV-NIS therapy had strong baseline T-cell responses both to measles virus proteins and to eight of the ten tested TAAs. Our data demonstrate that oncolytic virotherapy can function as an antigen agnostic vaccine, increasing cytotoxic T-lymphocyte responses against TAAs in patients with multiple myeloma, providing a basis for continued exploration of this modality in combination with immune checkpoint blockade.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies.Gene Ther. 2007 Feb;14(4):324-33. doi: 10.1038/sj.gt.3302880. Epub 2006 Oct 19. Gene Ther. 2007. PMID: 17051248
-
Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma.Leukemia. 2017 Dec;31(12):2791-2798. doi: 10.1038/leu.2017.120. Epub 2017 Apr 25. Leukemia. 2017. PMID: 28439108 Free PMC article. Clinical Trial.
-
Preclinical efficacy of the oncolytic measles virus expressing the sodium iodide symporter in iodine non-avid anaplastic thyroid cancer: a novel therapeutic agent allowing noninvasive imaging and radioiodine therapy.Cancer Gene Ther. 2012 Sep;19(9):659-65. doi: 10.1038/cgt.2012.47. Epub 2012 Jul 13. Cancer Gene Ther. 2012. PMID: 22790962
-
Oncolytic Virotherapy: A Contest between Apples and Oranges.Mol Ther. 2017 May 3;25(5):1107-1116. doi: 10.1016/j.ymthe.2017.03.026. Epub 2017 Apr 6. Mol Ther. 2017. PMID: 28392162 Free PMC article. Review.
-
Measles to the Rescue: A Review of Oncolytic Measles Virus.Viruses. 2016 Oct 22;8(10):294. doi: 10.3390/v8100294. Viruses. 2016. PMID: 27782084 Free PMC article. Review.
Cited by
-
Research progress in inducing immunogenic cell death of tumor cells.Front Immunol. 2022 Nov 17;13:1017400. doi: 10.3389/fimmu.2022.1017400. eCollection 2022. Front Immunol. 2022. PMID: 36466838 Free PMC article. Review.
-
Immunomodulatory Arming Factors-The Current Paradigm for Oncolytic Vectors Relies on Immune Stimulating Molecules.Int J Mol Sci. 2021 Aug 22;22(16):9051. doi: 10.3390/ijms22169051. Int J Mol Sci. 2021. PMID: 34445759 Free PMC article. Review.
-
Current clinical landscape of oncolytic viruses as novel cancer immunotherapeutic and recent preclinical advancements.Front Immunol. 2022 Aug 25;13:953410. doi: 10.3389/fimmu.2022.953410. eCollection 2022. Front Immunol. 2022. PMID: 36091031 Free PMC article. Review.
-
Oncolytic viruses improve cancer immunotherapy by reprogramming solid tumor microenvironment.Med Oncol. 2023 Dec 8;41(1):8. doi: 10.1007/s12032-023-02233-0. Med Oncol. 2023. PMID: 38062315 Review.
-
Immunotherapy approaches for hematological cancers.iScience. 2022 Oct 10;25(11):105326. doi: 10.1016/j.isci.2022.105326. eCollection 2022 Nov 18. iScience. 2022. PMID: 36325064 Free PMC article. Review.
References
-
- Martino M, Postorino M, Gallo GA, Messina G, Neri S, Piro E, et al. Long-term results in multiple myeloma after high-dose melphalan and autologous transplantation according to response categories in the era of old drugs. Clin Lymphoma Myeloma Leuk. 2014;14:148–54. - PubMed
-
- Miyamoto T, Yoshimoto G, Kamimura T, Muta T, Takashima S, Ito Y, et al. Combination of high-dose melphalan and bortezomib as conditioning regimen for autologous peripheral blood stem cell transplantation in multiple myeloma. Int J Hematol. 2013;98:337–45. - PubMed
-
- Atanackovic D, Luetkens T, Hildebrandt Y, Arfsten J, Bartels K, Horn C, et al. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin Cancer Res. 2009;15:1343–52. - PubMed
-
- Jungbluth AA, Ely S, DiLiberto M, Niesvizky R, Williamson B, Frosina D, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–74. - PubMed
-
- Lulla P. Tumor-associated antigen-specific cytotoxic T-lymphocytes for multiple myeloma (TACTAM). 2018. https://clinicaltrials.gov/ct2/show/NCT02291848.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

