TNFAIP3 Deficiency Affects Monocytes, Monocytes-Derived Cells and Microglia in Mice
- PMID: 32325694
- PMCID: PMC7215837
- DOI: 10.3390/ijms21082830
TNFAIP3 Deficiency Affects Monocytes, Monocytes-Derived Cells and Microglia in Mice
Abstract
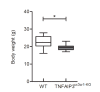
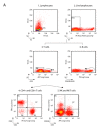
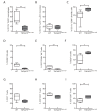
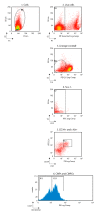
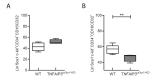
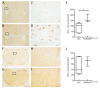
The intracellular-ubiquitin-ending-enzyme tumor necrosis factor alpha-induced protein 3 (TNFAIP3) is a potent inhibitor of the pro-inflammatory nuclear factor kappa-light-chain-enhancer of activated B cell (NF-kB) pathway. Single nucleotide polymorphisms in TNFAIP3 locus have been associated to autoimmune inflammatory disorders, including Multiple Sclerosis (MS). Previously, we reported a TNFAIP3 down-regulated gene expression level in blood and specifically in monocytes obtained from treatment-naïve MS patients compared to healthy controls (HC). Myeloid cells exert a key role in the pathogenesis of MS. Here we evaluated the effect of specific TNFAIP3 deficiency in myeloid cells including monocytes, monocyte-derived cells (M-MDC) and microglia analyzing lymphoid organs and microglia of mice. TNFAIP3 deletion is induced using conditional knock-out mice for myeloid lineage. Flow-cytometry and histological procedures were applied to assess the immune cell populations of spleen, lymph nodes and bone marrow and microglial cell density in the central nervous system (CNS), respectively. We found that TNFAIP3 deletion in myeloid cells induces a reduction in body weight, a decrease in the number of M-MDC and of common monocyte and granulocyte precursor cells (CMGPs). We also reported that the lack of TNFAIP3 in myeloid cells induces an increase in microglial cell density. The results suggest that TNFAIP3 in myeloid cells critically controls the development of M-MDC in lymphoid organ and of microglia in the CNS.
Keywords: TNFAIP3; inflammation; microglia; monocyte and macrophage; myeloid cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A20/Tumor Necrosis Factor α-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models.Front Immunol. 2018 Feb 21;9:104. doi: 10.3389/fimmu.2018.00104. eCollection 2018. Front Immunol. 2018. PMID: 29515565 Free PMC article. Review.
-
Unique primed status of microglia under the systemic autoimmune condition of lupus-prone mice.Arthritis Res Ther. 2019 Dec 30;21(1):303. doi: 10.1186/s13075-019-2067-8. Arthritis Res Ther. 2019. PMID: 31888754 Free PMC article.
-
House dust mite-driven neutrophilic airway inflammation in mice with TNFAIP3-deficient myeloid cells is IL-17-independent.Clin Exp Allergy. 2018 Dec;48(12):1705-1714. doi: 10.1111/cea.13262. Epub 2018 Sep 27. Clin Exp Allergy. 2018. PMID: 30171721
-
Monocyte/macrophage lineage commitment and distribution are affected by the lack of regulatory T cells in scurfy mice.Eur J Immunol. 2016 Jul;46(7):1656-68. doi: 10.1002/eji.201546200. Epub 2016 May 27. Eur J Immunol. 2016. PMID: 27130185
-
Do not judge a cell by its cover--diversity of CNS resident, adjoining and infiltrating myeloid cells in inflammation.Semin Immunopathol. 2015 Nov;37(6):591-605. doi: 10.1007/s00281-015-0520-6. Epub 2015 Aug 7. Semin Immunopathol. 2015. PMID: 26251238 Review.
Cited by
-
A Model of iPSC-Derived Macrophages with TNFAIP3 Overexpression Reveals the Peculiarities of TNFAIP3 Protein Expression and Function in Human Macrophages.Int J Mol Sci. 2023 Aug 16;24(16):12868. doi: 10.3390/ijms241612868. Int J Mol Sci. 2023. PMID: 37629049 Free PMC article.
-
Overexpression of the ubiquitin-editing enzyme A20 in the brain lesions of Multiple Sclerosis patients: moving from systemic to central nervous system inflammation.Brain Pathol. 2021 Mar;31(2):283-296. doi: 10.1111/bpa.12906. Epub 2020 Nov 18. Brain Pathol. 2021. PMID: 33051914 Free PMC article.
-
A role for misaligned gene expression of fetal gene program in the loss of female-specific cardiovascular protection in young obese and diabetic females.Front Endocrinol (Lausanne). 2023 Feb 23;14:1108449. doi: 10.3389/fendo.2023.1108449. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36909327 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

