Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial
- PMID: 32325038
- PMCID: PMC7172901
- DOI: 10.1016/S1473-3099(20)30160-2
Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial
Erratum in
-
Correction to Lancet Infect Dis 2019; published online April 20. https://doi.org/10.1016/S1473-3099(20)30160-2.Lancet Infect Dis. 2020 Jul;20(7):e148. doi: 10.1016/S1473-3099(20)30393-5. Epub 2020 May 12. Lancet Infect Dis. 2020. PMID: 32405264 Free PMC article. No abstract available.
-
Correction to Lancet Infect Dis 2019; published online April 20. https://doi.org/10.1016/S1473-3099(20)30160-2.Lancet Infect Dis. 2020 Jul;20(7):e148. doi: 10.1016/S1473-3099(20)30508-9. Epub 2020 Jun 8. Lancet Infect Dis. 2020. PMID: 32526192 Free PMC article. No abstract available.
Abstract
Background: Cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection continue to rise in the Arabian Peninsula 7 years after it was first described in Saudi Arabia. MERS-CoV poses a significant risk to public health security because of an absence of currently available effective countermeasures. We aimed to assess the safety and immunogenicity of the candidate simian adenovirus-vectored vaccine expressing the full-length spike surface glycoprotein, ChAdOx1 MERS, in humans.
Methods: This dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial was done at the Centre for Clinical Vaccinology and Tropical Medicine (Oxford, UK) and included healthy people aged 18-50 years with negative pre-vaccination tests for HIV antibodies, hepatitis B surface antigen, and hepatitis C antibodies (and a negative urinary pregnancy test for women). Participants received a single intramuscular injection of ChAdOx1 MERS at three different doses: the low-dose group received 5 × 109 viral particles, the intermediate-dose group received 2·5 × 1010 viral particles, and the high-dose group received 5 × 1010 viral particles. The primary objective was to assess safety and tolerability of ChAdOx1 MERS, measured by the occurrence of solicited, unsolicited, and serious adverse events after vaccination. The secondary objective was to assess the cellular and humoral immunogenicity of ChAdOx1 MERS, measured by interferon-γ-linked enzyme-linked immunospot, ELISA, and virus neutralising assays after vaccination. Participants were followed up for up to 12 months. This study is registered with ClinicalTrials.gov, NCT03399578.
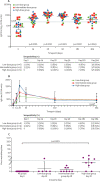
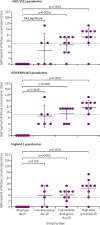
Findings: Between March 14 and Aug 15, 2018, 24 participants were enrolled: six were assigned to the low-dose group, nine to the intermediate-dose group, and nine to the high-dose group. All participants were available for follow-up at 6 months, but five (one in the low-dose group, one in the intermediate-dose group, and three in the high-dose group) were lost to follow-up at 12 months. A single dose of ChAdOx1 MERS was safe at doses up to 5 × 1010 viral particles with no vaccine-related serious adverse events reported by 12 months. One serious adverse event reported was deemed to be not related to ChAdOx1 MERS. 92 (74% [95% CI 66-81]) of 124 solicited adverse events were mild, 31 (25% [18-33]) were moderate, and all were self-limiting. Unsolicited adverse events in the 28 days following vaccination considered to be possibly, probably, or definitely related to ChAdOx1 MERS were predominantly mild in nature and resolved within the follow-up period of 12 months. The proportion of moderate and severe adverse events was significantly higher in the high-dose group than in the intermediate-dose group (relative risk 5·83 [95% CI 2·11-17·42], p<0·0001) Laboratory adverse events considered to be at least possibly related to the study intervention were self-limiting and predominantly mild in severity. A significant increase from baseline in T-cell (p<0·003) and IgG (p<0·0001) responses to the MERS-CoV spike antigen was observed at all doses. Neutralising antibodies against live MERS-CoV were observed in four (44% [95% CI 19-73]) of nine participants in the high-dose group 28 days after vaccination, and 19 (79% [58-93]) of 24 participants had antibodies capable of neutralisation in a pseudotyped virus neutralisation assay.
Interpretation: ChAdOx1 MERS was safe and well tolerated at all tested doses. A single dose was able to elicit both humoral and cellular responses against MERS-CoV. The results of this first-in-human clinical trial support clinical development progression into field phase 1b and 2 trials.
Funding: UK Department of Health and Social Care, using UK Aid funding, managed by the UK National Institute for Health Research.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial.Lancet Infect Dis. 2020 Jul;20(7):827-838. doi: 10.1016/S1473-3099(20)30248-6. Epub 2020 Apr 21. Lancet Infect Dis. 2020. PMID: 32325037 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial.Lancet. 2020 Aug 15;396(10249):467-478. doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Lancet. 2020. PMID: 32702298 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults (MERS002): an open-label, non-randomised, dose-escalation, phase 1b trial.Lancet Microbe. 2022 Jan;3(1):e11-e20. doi: 10.1016/S2666-5247(21)00193-2. Epub 2021 Nov 3. Lancet Microbe. 2022. PMID: 34751259 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial.Lancet Infect Dis. 2019 Sep;19(9):1013-1022. doi: 10.1016/S1473-3099(19)30266-X. Epub 2019 Jul 24. Lancet Infect Dis. 2019. PMID: 31351922 Free PMC article. Clinical Trial.
-
Immunogenicity and Safety of Chikungunya Vaccines: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2024 Aug 27;12(9):969. doi: 10.3390/vaccines12090969. Vaccines (Basel). 2024. PMID: 39340001 Free PMC article. Review.
Cited by
-
Coronavirus vaccines leap through safety trials - but which will work is anybody's guess.Nature. 2020 Jul;583(7818):669-670. doi: 10.1038/d41586-020-02174-y. Nature. 2020. PMID: 32710003 No abstract available.
-
Optimising T cell (re)boosting strategies for adenoviral and modified vaccinia Ankara vaccine regimens in humans.NPJ Vaccines. 2020 Oct 12;5:94. doi: 10.1038/s41541-020-00240-0. eCollection 2020. NPJ Vaccines. 2020. PMID: 33083029 Free PMC article.
-
A Comprehensive Investigation Regarding the Differentiation of the Procurable COVID-19 Vaccines.AAPS PharmSciTech. 2022 Mar 21;23(4):95. doi: 10.1208/s12249-022-02247-3. AAPS PharmSciTech. 2022. PMID: 35314902 Free PMC article. Review.
-
Computational Design and Experimental Evaluation of MERS-CoV siRNAs in Selected Cell Lines.Diagnostics (Basel). 2023 Jan 2;13(1):151. doi: 10.3390/diagnostics13010151. Diagnostics (Basel). 2023. PMID: 36611443 Free PMC article.
-
Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome.Nat Commun. 2022 Jul 19;13(1):4182. doi: 10.1038/s41467-022-31557-0. Nat Commun. 2022. PMID: 35853863 Free PMC article.
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. - PubMed
-
- WHO MERS situation update. January 2020. http://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html
-
- WHO . World Health Organization; Geneva: 2018. WHO MERS global summary and assessment of risk.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

