Neoadjuvant Nivolumab for Patients With Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial
- PMID: 32324435
- PMCID: PMC7392746
- DOI: 10.1200/JCO.20.00201
Neoadjuvant Nivolumab for Patients With Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial
Abstract
Purpose: Merkel cell carcinoma (MCC) is a rare, aggressive skin cancer commonly driven by the Merkel cell polyomavirus (MCPyV). The programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) immunosuppressive pathway is often upregulated in MCC, and advanced metastatic MCC frequently responds to PD-1 blockade. We report what we believe to be the first trial of anti-PD-1 in the neoadjuvant setting for resectable MCC.
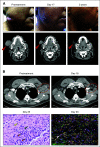
Methods: In the phase I/II CheckMate 358 study of virus-associated cancer types, patients with resectable MCC received nivolumab 240 mg intravenously on days 1 and 15. Surgery was planned on day 29. Tumor regression was assessed radiographically and microscopically. Tumor MCPyV status, PD-L1 expression, and tumor mutational burden (TMB) were assessed in pretreatment tumor biopsies.
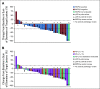
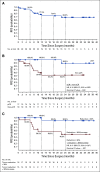
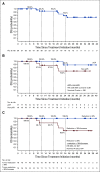
Results: Thirty-nine patients with American Joint Committee on Cancer stage IIA-IV resectable MCC received ≥ 1 nivolumab dose. Three patients (7.7%) did not undergo surgery because of tumor progression (n = 1) or adverse events (n = 2). Any-grade treatment-related adverse events occurred in 18 patients (46.2%), and grade 3-4 events in 3 patients (7.7%), with no unexpected toxicities. Among 36 patients who underwent surgery, 17 (47.2%) achieved a pathologic complete response (pCR). Among 33 radiographically evaluable patients who underwent surgery, 18 (54.5%) had tumor reductions ≥ 30%. Responses were observed regardless of tumor MCPyV, PD-L1, or TMB status. At a median follow-up of 20.3 months, median recurrence-free survival (RFS) and overall survival were not reached. RFS significantly correlated with pCR and radiographic response at the time of surgery. No patient with a pCR had tumor relapse during observation.
Conclusion: Nivolumab administered approximately 4 weeks before surgery in MCC was generally tolerable and induced pCRs and radiographic tumor regressions in approximately one half of treated patients. These early markers of response significantly predicted improved RFS. Additional investigation of these promising findings is warranted.
Trial registration: ClinicalTrials.gov NCT02488759.
Figures
Similar articles
-
The Genomic Landscape of Merkel Cell Carcinoma and Clinicogenomic Biomarkers of Response to Immune Checkpoint Inhibitor Therapy.Clin Cancer Res. 2019 Oct 1;25(19):5961-5971. doi: 10.1158/1078-0432.CCR-18-4159. Epub 2019 Aug 9. Clin Cancer Res. 2019. PMID: 31399473 Free PMC article.
-
Immunotherapy for Merkel Cell Carcinoma.Curr Treat Options Oncol. 2018 Sep 20;19(11):57. doi: 10.1007/s11864-018-0574-5. Curr Treat Options Oncol. 2018. PMID: 30238195 Review.
-
First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers.J Clin Oncol. 2019 Apr 20;37(12):992-1000. doi: 10.1200/JCO.18.01042. Epub 2019 Feb 20. J Clin Oncol. 2019. PMID: 30785829 Free PMC article. Clinical Trial.
-
Adjuvant immunotherapy with nivolumab versus observation in completely resected Merkel cell carcinoma (ADMEC-O): disease-free survival results from a randomised, open-label, phase 2 trial.Lancet. 2023 Sep 2;402(10404):798-808. doi: 10.1016/S0140-6736(23)00769-9. Epub 2023 Jul 11. Lancet. 2023. PMID: 37451295 Clinical Trial.
-
Immune Checkpoint Inhibitors and Beyond: An Overview of Immune-Based Therapies in Merkel Cell Carcinoma.Am J Clin Dermatol. 2019 Jun;20(3):391-407. doi: 10.1007/s40257-019-00427-9. Am J Clin Dermatol. 2019. PMID: 30784027 Review.
Cited by
-
Immunotherapy response induces divergent tertiary lymphoid structure morphologies in hepatocellular carcinoma.Nat Immunol. 2024 Nov;25(11):2110-2123. doi: 10.1038/s41590-024-01992-w. Epub 2024 Oct 25. Nat Immunol. 2024. PMID: 39455893
-
Targeted Drug Delivery in Periorbital Non-Melanocytic Skin Malignancies.Bioengineering (Basel). 2024 Oct 15;11(10):1029. doi: 10.3390/bioengineering11101029. Bioengineering (Basel). 2024. PMID: 39451404 Free PMC article. Review.
-
Intratumoral STING agonist reverses immune evasion in PD-(L)1-refractory Merkel cell carcinoma: mechanistic insights from detailed biomarker analyses.J Immunother Cancer. 2024 Oct 14;12(10):e009803. doi: 10.1136/jitc-2024-009803. J Immunother Cancer. 2024. PMID: 39401968 Free PMC article.
-
Pembrolizumab for the First-Line Treatment of Recurrent Locally Advanced or Metastatic Merkel Cell Carcinoma: Results from the Single-Arm, Open-Label, Phase III KEYNOTE-913 Study.Am J Clin Dermatol. 2024 Nov;25(6):987-996. doi: 10.1007/s40257-024-00885-w. Epub 2024 Oct 8. Am J Clin Dermatol. 2024. PMID: 39377880 Free PMC article. Clinical Trial.
-
Emerging Indications for Neoadjuvant Systemic Therapies in Cutaneous Malignancies.Med Sci (Basel). 2024 Jul 23;12(3):35. doi: 10.3390/medsci12030035. Med Sci (Basel). 2024. PMID: 39189198 Free PMC article. Review.
References
-
- Wong SQ, Waldeck K, Vergara IA, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75:5228–5234. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

