Methods for mechanical delivery of viral vectors into rhesus monkey brain
- PMID: 32302596
- PMCID: PMC7238764
- DOI: 10.1016/j.jneumeth.2020.108730
Methods for mechanical delivery of viral vectors into rhesus monkey brain
Abstract
Background: Modern molecular tools make it possible to manipulate neural activity in a reversible and cell-type specific manner. For rhesus monkey research, molecular tools are generally introduced via viral vectors. New instruments designed specifically for use in monkey research are needed to enhance the efficiency and reliability of vector delivery.
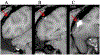
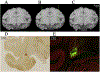
New method: A suite of multi-channel injection devices was developed to permit efficient and uniform vector delivery to cortical regions of the monkey brain. Manganese was co-infused with virus to allow rapid post-surgical confirmation of targeting accuracy using MRI. A needle guide was designed to increase the accuracy of sub-cortical targeting using stereotaxic co-ordinates.
Results: The multi-channel injection devices produced dense, uniform coverage of dorsal surface cortex, ventral surface cortex, and intra-sulcal cortex, respectively. Co-infusion of manganese with the viral vector allowed for immediate verification of injection accuracy. The needle guide improved accuracy of targeting sub-cortical structures by preventing needle deflection.
Comparison with existing method(s): The current methods, hand-held injections or single slow mechanical injection, for surface cortex transduction do not, in our hands, produce the density and uniformity of coverage provided by the injector arrays and associated infusion protocol.
Conclusions: The efficiency and reliability of vector delivery has been considerably improved by the development of new methods and instruments. This development should facilitate the translation of chemo- and optogenetic studies performed in smaller animals to larger animals such as rhesus monkeys.
Keywords: Chemogenetic; DREADD; Lentivirus; Non-human primates; Optogenetic; Rhesus monkey.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest No competing interests declared
Figures



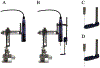

Similar articles
-
Convection Enhanced Delivery of Optogenetic Adeno-associated Viral Vector to the Cortex of Rhesus Macaque Under Guidance of Online MRI Images.J Vis Exp. 2019 May 23;(147):10.3791/59232. doi: 10.3791/59232. J Vis Exp. 2019. PMID: 31180352 Free PMC article.
-
Comparative study of the transfection efficiency of commonly used viral vectors in rhesus monkey (Macaca mulatta) brains.Zool Res. 2017 Mar 18;38(2):88-95. doi: 10.24272/j.issn.2095-8137.2017.015. Zool Res. 2017. PMID: 28409504 Free PMC article.
-
Surgical Procedure for Implantation of Opto-Array in Nonhuman Primates.Curr Protoc. 2023 Mar;3(3):e704. doi: 10.1002/cpz1.704. Curr Protoc. 2023. PMID: 36912623 Free PMC article.
-
Multifunctional Fibers as Tools for Neuroscience and Neuroengineering.Acc Chem Res. 2018 Apr 17;51(4):829-838. doi: 10.1021/acs.accounts.7b00558. Epub 2018 Mar 21. Acc Chem Res. 2018. PMID: 29561583 Review.
-
Advances in optogenetic and chemogenetic methods to study brain circuits in non-human primates.J Neural Transm (Vienna). 2018 Mar;125(3):547-563. doi: 10.1007/s00702-017-1697-8. Epub 2017 Feb 25. J Neural Transm (Vienna). 2018. PMID: 28238201 Free PMC article. Review.
Cited by
-
Direct Comparison of Epifluorescence and Immunostaining for Assessing Viral Mediated Gene Expression in the Primate Brain.Hum Gene Ther. 2023 Mar;34(5-6):228-246. doi: 10.1089/hum.2022.194. Epub 2023 Mar 7. Hum Gene Ther. 2023. PMID: 36719771 Free PMC article.
-
Image-dependence of the detectability of optogenetic stimulation in macaque inferotemporal cortex.Curr Biol. 2023 Feb 6;33(3):581-588.e4. doi: 10.1016/j.cub.2022.12.021. Epub 2023 Jan 6. Curr Biol. 2023. PMID: 36610394 Free PMC article.
-
Chemogenetic Tools and their Use in Studies of Neuropsychiatric Disorders.Physiol Res. 2024 Aug 30;73(S1):S449-S470. doi: 10.33549/physiolres.935401. Epub 2024 Jul 2. Physiol Res. 2024. PMID: 38957949 Free PMC article. Review.
-
Injections of AAV Vectors for Optogenetics in Anesthetized and Awake Behaving Non-Human Primate Brain.J Vis Exp. 2021 Aug 4;(174):10.3791/62546. doi: 10.3791/62546. J Vis Exp. 2021. PMID: 34424236 Free PMC article.
-
Evaluation of [18F]fluoroestradiol and ChRERα as a gene expression PET reporter system in rhesus monkey brain.Mol Ther. 2024 Jul 3;32(7):2223-2231. doi: 10.1016/j.ymthe.2024.05.031. Epub 2024 May 24. Mol Ther. 2024. PMID: 38796702
References
-
- Allen DC, Carlson TL, Xiong Y, Jin J, Grant KA, & Cuzon Carlson VC (2019). A Comparative Study of the Pharmacokinetics of Clozapine N-Oxide and Clozapine N-Oxide Hydrochloride Salt in Rhesus Macaques. Journal of Pharmacology and Experimental Therapeutics, 368(2), 199 10.1124/jpet.118.252031 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

