Targeting cancer stem cell pathways for cancer therapy
- PMID: 32296030
- PMCID: PMC7005297
- DOI: 10.1038/s41392-020-0110-5
Targeting cancer stem cell pathways for cancer therapy
Abstract
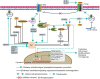
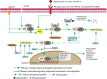
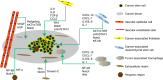
Since cancer stem cells (CSCs) were first identified in leukemia in 1994, they have been considered promising therapeutic targets for cancer therapy. These cells have self-renewal capacity and differentiation potential and contribute to multiple tumor malignancies, such as recurrence, metastasis, heterogeneity, multidrug resistance, and radiation resistance. The biological activities of CSCs are regulated by several pluripotent transcription factors, such as OCT4, Sox2, Nanog, KLF4, and MYC. In addition, many intracellular signaling pathways, such as Wnt, NF-κB (nuclear factor-κB), Notch, Hedgehog, JAK-STAT (Janus kinase/signal transducers and activators of transcription), PI3K/AKT/mTOR (phosphoinositide 3-kinase/AKT/mammalian target of rapamycin), TGF (transforming growth factor)/SMAD, and PPAR (peroxisome proliferator-activated receptor), as well as extracellular factors, such as vascular niches, hypoxia, tumor-associated macrophages, cancer-associated fibroblasts, cancer-associated mesenchymal stem cells, extracellular matrix, and exosomes, have been shown to be very important regulators of CSCs. Molecules, vaccines, antibodies, and CAR-T (chimeric antigen receptor T cell) cells have been developed to specifically target CSCs, and some of these factors are already undergoing clinical trials. This review summarizes the characterization and identification of CSCs, depicts major factors and pathways that regulate CSC development, and discusses potential targeted therapy for CSCs.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Cancer stem cells (CSCs) in cancer progression and therapy.J Cell Physiol. 2019 Jun;234(6):8381-8395. doi: 10.1002/jcp.27740. Epub 2018 Nov 11. J Cell Physiol. 2019. PMID: 30417375 Review.
-
Emerging agents that target signaling pathways to eradicate colorectal cancer stem cells.Cancer Commun (Lond). 2021 Dec;41(12):1275-1313. doi: 10.1002/cac2.12235. Epub 2021 Nov 17. Cancer Commun (Lond). 2021. PMID: 34791817 Free PMC article. Review.
-
Oncofetal proteins and cancer stem cells.Essays Biochem. 2022 Sep 16;66(4):423-433. doi: 10.1042/EBC20220025. Essays Biochem. 2022. PMID: 35670043 Review.
-
The oncogenic potential of NANOG: An important cancer induction mediator.J Cell Physiol. 2021 Apr;236(4):2443-2458. doi: 10.1002/jcp.30063. Epub 2020 Sep 22. J Cell Physiol. 2021. PMID: 32960465 Review.
-
Signaling pathways and microRNAs, the orchestrators of NANOG activity during cancer induction.Life Sci. 2020 Nov 1;260:118337. doi: 10.1016/j.lfs.2020.118337. Epub 2020 Aug 22. Life Sci. 2020. PMID: 32841661 Review.
Cited by
-
The influence of biophysical niche on tumor-associated macrophages in liver cancer.Hepatol Commun. 2024 Oct 30;8(11):e0569. doi: 10.1097/HC9.0000000000000569. eCollection 2024 Nov 1. Hepatol Commun. 2024. PMID: 39470328 Free PMC article. Review.
-
Role of a Polyphenol-Enriched Blueberry Preparation on Inhibition of Melanoma Cancer Stem Cells and Modulation of MicroRNAs.Biomedicines. 2024 Jan 16;12(1):193. doi: 10.3390/biomedicines12010193. Biomedicines. 2024. PMID: 38255297 Free PMC article.
-
Stem Cell Theory of Cancer: Clinical Implications of Epigenomic versus Genomic Biomarkers in Cancer Care.Cancers (Basel). 2023 Nov 22;15(23):5533. doi: 10.3390/cancers15235533. Cancers (Basel). 2023. PMID: 38067237 Free PMC article.
-
Adipose-derived mesenchymal stem cells promote the malignant phenotype of cervical cancer.Sci Rep. 2020 Aug 26;10(1):14205. doi: 10.1038/s41598-020-69907-x. Sci Rep. 2020. PMID: 32848147 Free PMC article.
-
Intricate relationship between cancer stemness, metastasis, and drug resistance.MedComm (2020). 2024 Sep 21;5(10):e710. doi: 10.1002/mco2.710. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39309691 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

