Proinflammatory P2Y14 receptor inhibition protects against ischemic acute kidney injury in mice
- PMID: 32287042
- PMCID: PMC7324186
- DOI: 10.1172/JCI134791
Proinflammatory P2Y14 receptor inhibition protects against ischemic acute kidney injury in mice
Abstract
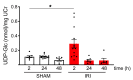
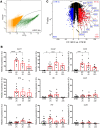
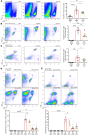
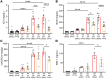
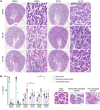
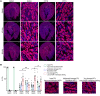
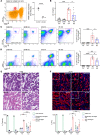
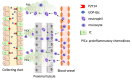
Ischemic acute kidney injury (AKI), a complication that frequently occurs in hospital settings, is often associated with hemodynamic compromise, sepsis, cardiac surgery, or exposure to nephrotoxins. Here, using a murine renal ischemia/reperfusion injury (IRI) model, we show that intercalated cells (ICs) rapidly adopted a proinflammatory phenotype after IRI. Wwe demonstrate that during the early phase of AKI either blockade of the proinflammatory P2Y14 receptor located on the apical membrane of ICs or ablation of the gene encoding the P2Y14 receptor in ICs (a) inhibited IRI-induced increase of chemokine expression in ICs, (b) reduced neutrophil and monocyte renal infiltration, (c) reduced the extent of kidney dysfunction, and (d) attenuated proximal tubule damage. These observations indicate that the P2Y14 receptor participates in the very first inflammatory steps associated with ischemic AKI. In addition, we show that the concentration of the P2Y14 receptor ligand UDP-glucose (UDP-Glc) was higher in urine samples from intensive care unit patients who developed AKI compared with patients without AKI. In particular, we observed a strong correlation between UDP-Glc concentration and the development of AKI in cardiac surgery patients. Our study identifies the UDP-Glc/P2Y14 receptor axis as a potential target for the prevention and/or attenuation of ischemic AKI.
Keywords: Cellular immune response; Inflammation; Monocytes; Nephrology; Neutrophils.
Conflict of interest statement
Figures

Comment in
-
Targeting inflammation in AKI by P2Y14 receptor inhibition in mice.Nat Rev Nephrol. 2020 Jul;16(7):372. doi: 10.1038/s41581-020-0300-y. Nat Rev Nephrol. 2020. PMID: 32372063 No abstract available.
Similar articles
-
Unexpected Participation of Intercalated Cells in Renal Inflammation and Acute Kidney Injury.Nephron. 2022;146(3):268-273. doi: 10.1159/000519265. Epub 2021 Oct 15. Nephron. 2022. PMID: 34657041 Free PMC article. Review.
-
Renal intercalated cells sense and mediate inflammation via the P2Y14 receptor.PLoS One. 2015 Mar 23;10(3):e0121419. doi: 10.1371/journal.pone.0121419. eCollection 2015. PLoS One. 2015. PMID: 25799465 Free PMC article.
-
Pre-ischemic renal lavage protects against renal ischemia-reperfusion injury by attenuation of local and systemic inflammatory responses.FASEB J. 2020 Dec;34(12):16307-16318. doi: 10.1096/fj.201902943R. Epub 2020 Oct 21. FASEB J. 2020. PMID: 33089923
-
Geniposide attenuates inflammatory response by suppressing P2Y14 receptor and downstream ERK1/2 signaling pathway in oxygen and glucose deprivation-induced brain microvascular endothelial cells.J Ethnopharmacol. 2016 Jun 5;185:77-86. doi: 10.1016/j.jep.2016.03.025. Epub 2016 Mar 11. J Ethnopharmacol. 2016. PMID: 26976766
-
Novel Proinflammatory Function of Renal Intercalated Cells.Ann Nutr Metab. 2018;72 Suppl 2:11-16. doi: 10.1159/000488303. Epub 2018 Jun 20. Ann Nutr Metab. 2018. PMID: 29925073 Review.
Cited by
-
Landscape of GPCR expression along the mouse nephron.Am J Physiol Renal Physiol. 2021 Jul 1;321(1):F50-F68. doi: 10.1152/ajprenal.00077.2021. Epub 2021 May 24. Am J Physiol Renal Physiol. 2021. PMID: 34029142 Free PMC article.
-
Structure-Activity Relationship of Heterocyclic P2Y14 Receptor Antagonists: Removal of the Zwitterionic Character with Piperidine Bioisosteres.J Med Chem. 2021 Apr 22;64(8):5099-5122. doi: 10.1021/acs.jmedchem.1c00164. Epub 2021 Mar 31. J Med Chem. 2021. PMID: 33787273 Free PMC article.
-
Immunoregulatory mechanisms between epithelial clear cells and mononuclear phagocytes in the epididymis.Andrology. 2024 Jul;12(5):949-963. doi: 10.1111/andr.13509. Epub 2023 Aug 12. Andrology. 2024. PMID: 37572347 Review.
-
P2Y14 Receptor Antagonists Reverse Chronic Neuropathic Pain in a Mouse Model.ACS Med Chem Lett. 2020 Apr 30;11(6):1281-1286. doi: 10.1021/acsmedchemlett.0c00115. eCollection 2020 Jun 11. ACS Med Chem Lett. 2020. PMID: 32551012 Free PMC article.
-
The Glycosyltransferase Pathway: An Integrated Analysis of the Cell Metabolome.Metabolites. 2022 Oct 21;12(10):1006. doi: 10.3390/metabo12101006. Metabolites. 2022. PMID: 36295907 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

