An intracellular membrane protein GEP1 regulates xanthurenic acid induced gametogenesis of malaria parasites
- PMID: 32273496
- PMCID: PMC7145802
- DOI: 10.1038/s41467-020-15479-3
An intracellular membrane protein GEP1 regulates xanthurenic acid induced gametogenesis of malaria parasites
Abstract
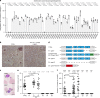
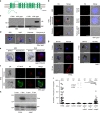
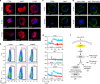
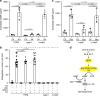
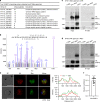
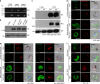
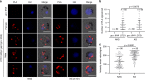
Gametocytes differentiation to gametes (gametogenesis) within mosquitos is essential for malaria parasite transmission. Both reduction in temperature and mosquito-derived XA or elevated pH are required for triggering cGMP/PKG dependent gametogenesis. However, the parasite molecule for sensing or transducing these environmental signals to initiate gametogenesis remains unknown. Here we perform a CRISPR/Cas9-based functional screening of 59 membrane proteins expressed in the gametocytes of Plasmodium yoelii and identify that GEP1 is required for XA-stimulated gametogenesis. GEP1 disruption abolishes XA-stimulated cGMP synthesis and the subsequent signaling and cellular events, such as Ca2+ mobilization, gamete formation, and gametes egress out of erythrocytes. GEP1 interacts with GCα, a cGMP synthesizing enzyme in gametocytes. Both GEP1 and GCα are expressed in cytoplasmic puncta of both male and female gametocytes. Depletion of GCα impairs XA-stimulated gametogenesis, mimicking the defect of GEP1 disruption. The identification of GEP1 being essential for gametogenesis provides a potential new target for intervention of parasite transmission.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Characterizing the Specific Recognition of Xanthurenic Acid by GEP1 and GEP1-GCα Interactions in cGMP Signaling Pathway in Gametogenesis of Malaria Parasites.Int J Mol Sci. 2023 Jan 29;24(3):2561. doi: 10.3390/ijms24032561. Int J Mol Sci. 2023. PMID: 36768882 Free PMC article.
-
A G-Protein-Coupled Receptor Modulates Gametogenesis via PKG-Mediated Signaling Cascade in Plasmodium berghei.Microbiol Spectr. 2022 Apr 27;10(2):e0015022. doi: 10.1128/spectrum.00150-22. Epub 2022 Apr 11. Microbiol Spectr. 2022. PMID: 35404079 Free PMC article.
-
Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase.PLoS Biol. 2008 Jun 3;6(6):e139. doi: 10.1371/journal.pbio.0060139. PLoS Biol. 2008. PMID: 18532880 Free PMC article.
-
cGMP homeostasis in malaria parasites-The key to perceiving and integrating environmental changes during transmission to the mosquito.Mol Microbiol. 2021 May;115(5):829-838. doi: 10.1111/mmi.14633. Epub 2020 Nov 21. Mol Microbiol. 2021. PMID: 33112460 Review.
-
Gametogenesis in Plasmodium: Delving Deeper to Connect the Dots.Front Cell Infect Microbiol. 2022 Jun 15;12:877907. doi: 10.3389/fcimb.2022.877907. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782151 Free PMC article. Review.
Cited by
-
Plasmodium transcription repressor AP2-O3 regulates sex-specific identity of gene expression in female gametocytes.EMBO Rep. 2021 May 5;22(5):e51660. doi: 10.15252/embr.202051660. Epub 2021 Mar 4. EMBO Rep. 2021. PMID: 33665945 Free PMC article.
-
An axonemal intron splicing program sustains Plasmodium male development.Nat Commun. 2024 Jun 1;15(1):4697. doi: 10.1038/s41467-024-49002-9. Nat Commun. 2024. PMID: 38824128 Free PMC article.
-
Updated List of Transport Proteins in Plasmodium falciparum.Front Cell Infect Microbiol. 2022 Jun 24;12:926541. doi: 10.3389/fcimb.2022.926541. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35811673 Free PMC article. Review.
-
Plasmodium falciparum Guanylyl Cyclase-Alpha and the Activity of Its Appended P4-ATPase Domain Are Essential for cGMP Synthesis and Blood-Stage Egress.mBio. 2021 Jan 26;12(1):e02694-20. doi: 10.1128/mBio.02694-20. mBio. 2021. PMID: 33500341 Free PMC article.
-
Characterizing the Specific Recognition of Xanthurenic Acid by GEP1 and GEP1-GCα Interactions in cGMP Signaling Pathway in Gametogenesis of Malaria Parasites.Int J Mol Sci. 2023 Jan 29;24(3):2561. doi: 10.3390/ijms24032561. Int J Mol Sci. 2023. PMID: 36768882 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

