Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients
- PMID: 32267220
- PMCID: PMC7323511
- DOI: 10.3201/eid2607.200841
Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients
Abstract
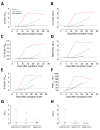
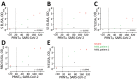
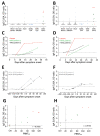
A new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has recently emerged to cause a human pandemic. Although molecular diagnostic tests were rapidly developed, serologic assays are still lacking, yet urgently needed. Validated serologic assays are needed for contact tracing, identifying the viral reservoir, and epidemiologic studies. We developed serologic assays for detection of SARS-CoV-2 neutralizing, spike protein-specific, and nucleocapsid-specific antibodies. Using serum samples from patients with PCR-confirmed SARS-CoV-2 infections, other coronaviruses, or other respiratory pathogenic infections, we validated and tested various antigens in different in-house and commercial ELISAs. We demonstrated that most PCR-confirmed SARS-CoV-2-infected persons seroconverted by 2 weeks after disease onset. We found that commercial S1 IgG or IgA ELISAs were of lower specificity, and sensitivity varied between the 2 assays; the IgA ELISA showed higher sensitivity. Overall, the validated assays described can be instrumental for detection of SARS-CoV-2-specific antibodies for diagnostic, seroepidemiologic, and vaccine evaluation studies.
Keywords: COVID-19; ELISA; HCoV; RBD; SARS-CoV-2; Severe acute respiratory syndrome coronavirus 2; antibodies; coronavirus; coronavirus disease 2019; human coronavirus; neutralization; nucleocapsid protein; receptor-binding domain; respiratory infections; serologic analysis; spike protein; viruses; zoonoses.
Figures


Comment in
-
The prevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) IgG antibodies in intensive care unit (ICU) healthcare personnel (HCP) and its implications-a single-center, prospective, pilot study.Infect Control Hosp Epidemiol. 2021 May;42(5):638-639. doi: 10.1017/ice.2020.298. Epub 2020 Jun 12. Infect Control Hosp Epidemiol. 2021. PMID: 32530392 Free PMC article. No abstract available.
-
Review of Current Advances in Serologic Testing for COVID-19.Am J Clin Pathol. 2020 Aug 5;154(3):293-304. doi: 10.1093/ajcp/aqaa112. Am J Clin Pathol. 2020. PMID: 32583852 Free PMC article.
Similar articles
-
Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays.J Clin Virol. 2020 Aug;129:104480. doi: 10.1016/j.jcv.2020.104480. Epub 2020 Jun 1. J Clin Virol. 2020. PMID: 32505777 Free PMC article.
-
Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity.JCI Insight. 2020 Sep 17;5(18):e142386. doi: 10.1172/jci.insight.142386. JCI Insight. 2020. PMID: 32796155 Free PMC article.
-
Clinical and Analytical Performance of an Automated Serological Test That Identifies S1/S2-Neutralizing IgG in COVID-19 Patients Semiquantitatively.J Clin Microbiol. 2020 Aug 24;58(9):e01224-20. doi: 10.1128/JCM.01224-20. Print 2020 Aug 24. J Clin Microbiol. 2020. PMID: 32580948 Free PMC article.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
The Role of Antibody Testing for SARS-CoV-2: Is There One?J Clin Microbiol. 2020 Jul 23;58(8):e00797-20. doi: 10.1128/JCM.00797-20. Print 2020 Jul 23. J Clin Microbiol. 2020. PMID: 32350047 Free PMC article. Review.
Cited by
-
Immunomodulatory effect of IFN-γ licensed adipose-mesenchymal stromal cells in an in vitro model of inflammation generated by SARS-CoV-2 antigens.Sci Rep. 2024 Oct 16;14(1):24235. doi: 10.1038/s41598-024-75776-5. Sci Rep. 2024. PMID: 39415027 Free PMC article.
-
Anti-SARS-CoV-2 IgM Antibody Levels Measured by an In-House ELISA in a Convalescent Latin Population Persist over Time and Exhibit Neutralizing Capacity to Several Variants of Concern.Diagnostics (Basel). 2024 Oct 3;14(19):2209. doi: 10.3390/diagnostics14192209. Diagnostics (Basel). 2024. PMID: 39410613 Free PMC article.
-
Utility of Extraction-Free SARS-CoV-2 Detection by RT-qPCR for COVID-19 Testing in a Resource-Limited Setting.Diseases. 2024 Aug 26;12(9):198. doi: 10.3390/diseases12090198. Diseases. 2024. PMID: 39329867 Free PMC article.
-
Systematic Study of Various Functionalization Steps for Ultrasensitive Detection of SARS-CoV-2 with Direct Laser-Functionalized Au-LIG Electrochemical Sensors.ACS Appl Mater Interfaces. 2024 Sep 18;16(37):49041-49052. doi: 10.1021/acsami.4c09571. Epub 2024 Sep 4. ACS Appl Mater Interfaces. 2024. PMID: 39231012 Free PMC article.
-
Evaluation of three alternative methods to the plaque reduction neutralizing assay for measuring neutralizing antibodies to dengue virus serotype 2.Virol J. 2024 Sep 3;21(1):208. doi: 10.1186/s12985-024-02459-y. Virol J. 2024. PMID: 39227969 Free PMC article.
References
-
- World Health Organization. Coronavirus disease (COVID-2019) situation reports [cited 2020 Mar 14]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

