Hydrogel Biomaterials for Application in Ocular Drug Delivery
- PMID: 32266248
- PMCID: PMC7099765
- DOI: 10.3389/fbioe.2020.00228
Hydrogel Biomaterials for Application in Ocular Drug Delivery
Abstract
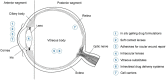
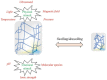
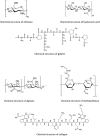
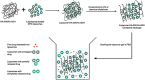
There are many challenges involved in ocular drug delivery. These are a result of the many tissue barriers and defense mechanisms that are present with the eye; such as the cornea, conjunctiva, the blinking reflex, and nasolacrimal drainage system. This leads to many of the conventional ophthalmic preparations, such as eye drops, having low bioavailability profiles, rapid removal from the administration site, and thus ineffective delivery of drugs. Hydrogels have been investigated as a delivery system which is able to overcome some of these challenges. These have been formulated as standalone systems or with the incorporation of other technologies such as nanoparticles. Hydrogels are able to be formulated in such a way that they are able to change from a liquid to gel as a response to a stimulus; known as "smart" or stimuli-responsive biotechnology platforms. Various different stimuli-responsive hydrogel systems are discussed in this article. Hydrogel drug delivery systems are able to be formulated from both synthetic and natural polymers, known as biopolymers. This review focuses on the formulations which incorporate biopolymers. These polymers have a number of benefits such as the fact that they are biodegradable, biocompatible, and non-cytotoxic. The biocompatibility of the polymers is essential for ocular drug delivery systems because the eye is an extremely sensitive organ which is known as an immune privileged site.
Keywords: biomaterials; biopolymers; hydrogel; nanotechnology; ocular drug delivery; safety by design.
Copyright © 2020 Lynch, Kondiah, Choonara, du Toit, Ally and Pillay.
Figures

Similar articles
-
Hydrogel-based ocular drug delivery systems for hydrophobic drugs.Eur J Pharm Sci. 2020 Nov 1;154:105503. doi: 10.1016/j.ejps.2020.105503. Epub 2020 Aug 1. Eur J Pharm Sci. 2020. PMID: 32745587 Review.
-
Exploring Hydrogel Nanoparticle Systems for Enhanced Ocular Drug Delivery.Gels. 2024 Sep 13;10(9):589. doi: 10.3390/gels10090589. Gels. 2024. PMID: 39330191 Free PMC article. Review.
-
Interpenetrating polymeric network (IPNs) in ophthalmic drug delivery: Breaking the barriers.Int Ophthalmol. 2023 Mar;43(3):1063-1074. doi: 10.1007/s10792-022-02482-4. Epub 2022 Sep 2. Int Ophthalmol. 2023. PMID: 36053474 Review.
-
Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy.Polymers (Basel). 2022 Jun 10;14(12):2359. doi: 10.3390/polym14122359. Polymers (Basel). 2022. PMID: 35745935 Free PMC article. Review.
-
Advanced Hydrogels Based Drug Delivery Systems for Ophthalmic Delivery.Recent Pat Drug Deliv Formul. 2019;13(4):291-300. doi: 10.2174/1872211314666200108094851. Recent Pat Drug Deliv Formul. 2019. PMID: 31912771 Review.
Cited by
-
Summary of the Therapeutic Options for Patients with Dry and Neovascular AMD.J Clin Med. 2024 Jul 19;13(14):4227. doi: 10.3390/jcm13144227. J Clin Med. 2024. PMID: 39064267 Free PMC article. Review.
-
Technological Advances in a Therapy of Primary Open-Angle Glaucoma: Insights into Current Nanotechnologies.J Clin Med. 2023 Sep 6;12(18):5798. doi: 10.3390/jcm12185798. J Clin Med. 2023. PMID: 37762739 Free PMC article. Review.
-
Emphasis on Nanostructured Lipid Carriers in the Ocular Delivery of Antibiotics.Pharm Nanotechnol. 2024;12(2):126-142. doi: 10.2174/2211738511666230727102213. Pharm Nanotechnol. 2024. PMID: 37519002 Review.
-
Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications.Polymers (Basel). 2021 Oct 31;13(21):3782. doi: 10.3390/polym13213782. Polymers (Basel). 2021. PMID: 34771338 Free PMC article. Review.
-
Breaking Barriers: Nanomedicine-Based Drug Delivery for Cataract Treatment.Int J Nanomedicine. 2024 May 6;19:4021-4040. doi: 10.2147/IJN.S463679. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38736657 Free PMC article. Review.
References
-
- Bain M. K., Bhowmik M., Ghosh S. H., Chattopadhyay D. (2009). In situ fast gelling formulation of methyl cellulose for in vitro ophthalmic controlled delivery of ketorolac tromethamine. J. Appl. Polymer Sci. 113 1241–1246. 10.1002/app.30040 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

