The Role of PD-1 in Acute and Chronic Infection
- PMID: 32265932
- PMCID: PMC7105608
- DOI: 10.3389/fimmu.2020.00487
The Role of PD-1 in Acute and Chronic Infection
Abstract
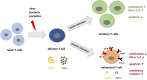
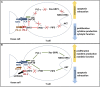
PD-1 as an immune checkpoint molecule down-regulates T cell activity during immune responses in order to prevent autoimmune tissue damage. In chronic infections or tumors, lasting antigen-exposure leads to permanent PD-1 expression that can limit immune-mediated clearance of pathogens or degenerated cells. Blocking PD-1 can enhance T cell function; in cancer treatment PD-1 blockade is already used as a successful therapy. However, the role of PD-1 expression and blocking in the context of acute and chronic infections is less defined. Building on its success in cancer therapy leads to the hypothesis that blocking PD-1 in infectious diseases is also beneficial in acute or chronic infections. This review will focus on the role of PD-1 expression in acute and chronic infections with virus, bacteria, and parasites, with a particular focus on recent studies regarding PD-1 blockade in infectious diseases.
Keywords: PD-1; PD-L1; PD-L2; T cell exhaustion; acute infection; checkpoint inhibitor; chronic infection; infectious disease.
Copyright © 2020 Jubel, Barbati, Burger, Wirtz and Schildberg.
Figures
Similar articles
-
Therapeutic intervention in cancer and chronic viral infections: antibody mediated manipulation of PD-1/PD-L1 interaction.Rev Recent Clin Trials. 2012 Feb;7(1):10-23. doi: 10.2174/157488712799363262. Rev Recent Clin Trials. 2012. PMID: 22023178 Review.
-
The Diverse Function of PD-1/PD-L Pathway Beyond Cancer.Front Immunol. 2019 Oct 4;10:2298. doi: 10.3389/fimmu.2019.02298. eCollection 2019. Front Immunol. 2019. PMID: 31636634 Free PMC article. Review.
-
PD-1 blockade improves Kupffer cell bacterial clearance in acute liver injury.J Clin Invest. 2021 Feb 15;131(4):e140196. doi: 10.1172/JCI140196. J Clin Invest. 2021. PMID: 33320839 Free PMC article.
-
Anti-PD-1/PD-L1 therapy for infectious diseases: learning from the cancer paradigm.Int J Infect Dis. 2017 Mar;56:221-228. doi: 10.1016/j.ijid.2017.01.028. Epub 2017 Feb 2. Int J Infect Dis. 2017. PMID: 28163164 Review.
-
Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1.J Leukoc Biol. 2016 Dec;100(6):1239-1254. doi: 10.1189/jlb.4HI0616-255R. Epub 2016 Sep 26. J Leukoc Biol. 2016. PMID: 27671246 Free PMC article.
Cited by
-
New Immunological Markers in Chromoblastomycosis-The Importance of PD-1 and PD-L1 Molecules in Human Infection.J Fungi (Basel). 2023 Dec 7;9(12):1172. doi: 10.3390/jof9121172. J Fungi (Basel). 2023. PMID: 38132773 Free PMC article.
-
Determinants of pegivirus persistence, cross-species infection, and adaptation in the laboratory mouse.PLoS Pathog. 2024 Aug 28;20(8):e1012436. doi: 10.1371/journal.ppat.1012436. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39196893 Free PMC article.
-
Sarcoidosis detected after COVID‑19 with T‑SPOT.TB positive: A case report.Exp Ther Med. 2023 Dec 18;27(2):67. doi: 10.3892/etm.2023.12355. eCollection 2024 Feb. Exp Ther Med. 2023. PMID: 38234612 Free PMC article.
-
Characterization of the immunosuppressive environment induced by larval Echinococcus granulosus during chronic experimental infection.Infect Immun. 2024 Feb 13;92(2):e0027623. doi: 10.1128/iai.00276-23. Epub 2024 Jan 4. Infect Immun. 2024. PMID: 38174942 Free PMC article.
-
Cutibacterium acnes Induces the Expression of Immunosuppressive Genes in Macrophages and is Associated with an Increase of Regulatory T-Cells in Prostate Cancer.Microbiol Spectr. 2021 Dec 22;9(3):e0149721. doi: 10.1128/spectrum.01497-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937192 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

