Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis
- PMID: 32251668
- PMCID: PMC7194936
- DOI: 10.1053/j.gastro.2020.03.065
Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis
Abstract
Background & aims: Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), which has been characterized by fever, respiratory, and gastrointestinal symptoms as well as shedding of virus RNA into feces. We performed a systematic review and meta-analysis of published gastrointestinal symptoms and detection of virus in stool and also summarized data from a cohort of patients with COVID-19 in Hong Kong.
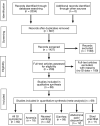
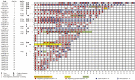
Methods: We collected data from the cohort of patients with COVID-19 in Hong Kong (N = 59; diagnosis from February 2 through February 29, 2020),and searched PubMed, Embase, Cochrane, and 3 Chinese databases through March 11, 2020, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We analyzed pooled data on the prevalence of overall and individual gastrointestinal symptoms (loss of appetite, nausea, vomiting, diarrhea, and abdominal pain or discomfort) using a random effects model.
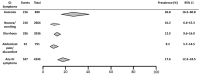
Results: Among the 59 patients with COVID-19 in Hong Kong, 15 patients (25.4%) had gastrointestinal symptoms, and 9 patients (15.3%) had stool that tested positive for virus RNA. Stool viral RNA was detected in 38.5% and 8.7% among those with and without diarrhea, respectively (P = .02). The median fecal viral load was 5.1 log10 copies per milliliter in patients with diarrhea vs 3.9 log10 copies per milliliter in patients without diarrhea (P = .06). In a meta-analysis of 60 studies comprising 4243 patients, the pooled prevalence of all gastrointestinal symptoms was 17.6% (95% confidence interval [CI], 12.3-24.5); 11.8% of patients with nonsevere COVID-19 had gastrointestinal symptoms (95% CI, 4.1-29.1), and 17.1% of patients with severe COVID-19 had gastrointestinal symptoms (95% CI, 6.9-36.7). In the meta-analysis, the pooled prevalence of stool samples that were positive for virus RNA was 48.1% (95% CI, 38.3-57.9); of these samples, 70.3% of those collected after loss of virus from respiratory specimens tested positive for the virus (95% CI, 49.6-85.1).
Conclusions: In an analysis of data from the Hong Kong cohort of patients with COVID-19 and a meta-analysis of findings from publications, we found that 17.6% of patients with COVID-19 had gastrointestinal symptoms. Virus RNA was detected in stool samples from 48.1% patients, even in stool collected after respiratory samples had negative test results. Health care workers should therefore exercise caution in collecting fecal samples or performing endoscopic procedures in patients with COVID-19, even during patient recovery.
Keywords: Fecal-to-Oral Transmission; PRISMA; SARS; Viral Persistence.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
SARS-CoV-2 RNA Detection in Gastrointestinal Sample Displays Poor Performance.Gastroenterology. 2021 Feb;160(3):972-973.e1. doi: 10.1053/j.gastro.2020.05.084. Epub 2020 Jun 13. Gastroenterology. 2021. PMID: 32544393 Free PMC article. No abstract available.
-
Reply.Gastroenterology. 2021 Feb;160(3):973-974. doi: 10.1053/j.gastro.2020.09.019. Epub 2020 Sep 22. Gastroenterology. 2021. PMID: 32971135 Free PMC article. No abstract available.
Similar articles
-
Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19.J Clin Virol. 2020 Jul;128:104386. doi: 10.1016/j.jcv.2020.104386. Epub 2020 Apr 29. J Clin Virol. 2020. PMID: 32388469 Free PMC article. Review.
-
Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Jun 1;3(6):e2011335. doi: 10.1001/jamanetworkopen.2020.11335. JAMA Netw Open. 2020. PMID: 32525549 Free PMC article.
-
The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients.J Med Virol. 2020 Jul;92(7):833-840. doi: 10.1002/jmv.25825. Epub 2020 Apr 25. J Med Virol. 2020. PMID: 32243607
-
Gastrointestinal symptoms and fecal nucleic acid testing of children with 2019 coronavirus disease: a systematic review and meta-analysis.Sci Rep. 2020 Oct 20;10(1):17846. doi: 10.1038/s41598-020-74913-0. Sci Rep. 2020. PMID: 33082472 Free PMC article.
-
Is SARS-CoV-2 Also an Enteric Pathogen With Potential Fecal-Oral Transmission? A COVID-19 Virological and Clinical Review.Gastroenterology. 2020 Jul;159(1):53-61. doi: 10.1053/j.gastro.2020.04.052. Epub 2020 Apr 27. Gastroenterology. 2020. PMID: 32353371 Free PMC article. Review.
Cited by
-
The first report on detecting SARS-CoV-2 inside bacteria of the human gut microbiome: A case series on asymptomatic family members and a child with COVID-19.F1000Res. 2024 Oct 16;11:135. doi: 10.12688/f1000research.77421.2. eCollection 2022. F1000Res. 2024. PMID: 39464247 Free PMC article.
-
Longitudinal wastewater-based surveillance of SARS-CoV-2 during 2023 in Ethiopia.Front Public Health. 2024 Oct 7;12:1394798. doi: 10.3389/fpubh.2024.1394798. eCollection 2024. Front Public Health. 2024. PMID: 39435409 Free PMC article.
-
Impact of Mild COVID-19 History on Oral-Gut Microbiota and Serum Metabolomics in Adult Patients with Crohn's Disease: Potential Beneficial Effects.Biomedicines. 2024 Sep 14;12(9):2103. doi: 10.3390/biomedicines12092103. Biomedicines. 2024. PMID: 39335616 Free PMC article.
-
Longitudinal fecal shedding of SARS-CoV-2, pepper mild mottle virus, and human mitochondrial DNA in COVID-19 patients.Front Med (Lausanne). 2024 Sep 11;11:1417967. doi: 10.3389/fmed.2024.1417967. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39323476 Free PMC article.
-
The comparison of pathogenicity among SARS-CoV-2 variants in domestic cats.Sci Rep. 2024 Sep 18;14(1):21815. doi: 10.1038/s41598-024-71791-8. Sci Rep. 2024. PMID: 39294189 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

