Using RWE research to extend clinical trials in diabetes: An example with implications for the future
- PMID: 32250529
- PMCID: PMC7216829
- DOI: 10.1111/dom.14021
Using RWE research to extend clinical trials in diabetes: An example with implications for the future
Abstract
Background: Although randomized, controlled trials (RCTs) are seen as the gold standard for evidence in clinical medicine, a number of considerations are increasing the use of real-world data (RWD) to generate evidence. A series of methodological challenges must be overcome in order for such real-world evidence (RWE) to gain acceptance. In diabetes, RWE faces some particular issues that have limited its development. As the natural history of diabetes progresses, patients' disease changes over time and treatments will be modified as a result. This evolving disease and treatment pattern requires application of methods that account for such changes over time. Research developing RWE in diabetes and other conditions has sometimes been subject to important biases, and researchers should be aware of, and take steps to mitigate potential for bias in order to enhance the evidence produced.
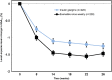
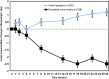
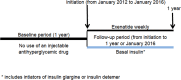
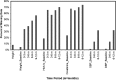
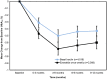
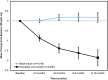
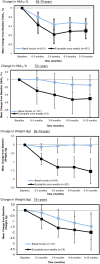
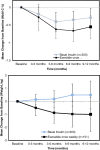
Results: We review a RWE study that replicated and extended evidence provided by a RCT regarding the effects of weekly exenatide relative to basal insulin (glargine or detemir) to illustrate a potential application of RWE. This study observed a 0.7% decrease in HbA1C for weekly exenatide relative to a 0.5% decrease in HbA1C for the comparator along with a 2 kg weight loss for weekly exenatide relative to a 0.25 kg weight gain, effects that were close to those from the RCT. Further, the RWE study was able to extend results to patient populations that were not well represented in the RCT.
Conclusion: Despite numerous challenges, RWE can be used to complement evidence from RCTs.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
Similar articles
-
The Case for Real-world Evidence in the Future of Clinical Research on Chronic Myeloid Leukemia.Clin Ther. 2019 Feb;41(2):336-349. doi: 10.1016/j.clinthera.2018.12.013. Epub 2019 Jan 30. Clin Ther. 2019. PMID: 30709609 Review.
-
An Attempt to Replicate Randomized Trials of Diabetes Treatments Using a Japanese Administrative Claims and Health Checkup Database: A Feasibility Study.Drugs Real World Outcomes. 2023 Jun;10(2):235-247. doi: 10.1007/s40801-023-00353-7. Epub 2023 Feb 1. Drugs Real World Outcomes. 2023. PMID: 36725811 Free PMC article.
-
Replication of Randomized, Controlled Trials Using Real-World Data: What Could Go Wrong?Value Health. 2021 Jan;24(1):112-115. doi: 10.1016/j.jval.2020.09.015. Epub 2020 Nov 20. Value Health. 2021. PMID: 33431143
-
The present and future scope of real-world evidence research in diabetes: What questions can and cannot be answered and what might be possible in the future?Diabetes Obes Metab. 2020 Apr;22 Suppl 3:21-34. doi: 10.1111/dom.13929. Diabetes Obes Metab. 2020. PMID: 32250528 Review.
-
Emulating Randomized Clinical Trials With Nonrandomized Real-World Evidence Studies: First Results From the RCT DUPLICATE Initiative.Circulation. 2021 Mar 9;143(10):1002-1013. doi: 10.1161/CIRCULATIONAHA.120.051718. Epub 2020 Dec 17. Circulation. 2021. PMID: 33327727 Free PMC article.
Cited by
-
Cardiovascular and Renal Effectiveness of GLP-1 Receptor Agonists vs. Other Glucose-Lowering Drugs in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Real-World Studies.Metabolites. 2022 Feb 15;12(2):183. doi: 10.3390/metabo12020183. Metabolites. 2022. PMID: 35208256 Free PMC article. Review.
-
Comparative effectiveness of dulaglutide versus liraglutide in Asian type 2 diabetes patients: a multi-institutional cohort study and meta-analysis.Cardiovasc Diabetol. 2020 Oct 9;19(1):172. doi: 10.1186/s12933-020-01148-8. Cardiovasc Diabetol. 2020. PMID: 33036617 Free PMC article.
-
Cost-effectiveness of GLP-1 receptor agonists versus insulin for the treatment of type 2 diabetes: a real-world study and systematic review.Cardiovasc Diabetol. 2021 Jan 19;20(1):21. doi: 10.1186/s12933-020-01211-4. Cardiovasc Diabetol. 2021. PMID: 33468131 Free PMC article.
-
A systematic review of health-related quality of life outcomes in patients with advanced breast cancer treated with palbociclib.J Comp Eff Res. 2024 Oct;13(10):e240111. doi: 10.57264/cer-2024-0111. Epub 2024 Sep 10. J Comp Eff Res. 2024. PMID: 39254990 Free PMC article.
-
Effectiveness in Real World of Once Weekly Semaglutide in People with Type 2 Diabetes: Glucagon-Like Peptide Receptor Agonist Naïve or Switchers from Other Glucagon-Like Peptide Receptor Agonists: Results from a Retrospective Observational Study in Umbria.Diabetes Ther. 2022 Mar;13(3):551-567. doi: 10.1007/s13300-022-01218-y. Epub 2022 Mar 1. Diabetes Ther. 2022. PMID: 35230650 Free PMC article.
References
-
- Sackett DL. Why did the randomized clinical trial become the primary focus of my career? Value Health. 2015;18:550‐552. - PubMed
-
- Bothwell LE, Podolsky SH. The emergence of the randomized, controlled trial. N Engl J Med. 2016;375:501‐504. - PubMed
-
- Berger ML, Sox H, Willke RJ, et al. Good practices for real‐world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR‐ISPE special task force on real‐world evidence in health care decision making. Pharmacoepidemiol Drug Saf. 2017;26:1033‐1039. - PMC - PubMed
-
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence ‐ what is it and what can it tell us? N Engl J Med. 2016;375:2293‐2297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

