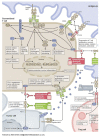
Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence
- PMID: 32245016
- PMCID: PMC7140028
- DOI: 10.3390/cancers12030738
Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence
Abstract
Cancer is associated with higher morbidity and mortality and is the second leading cause of death in the US. Further, in some nations, cancer has overtaken heart disease as the leading cause of mortality. Identification of molecular mechanisms by which cancerous cells evade T cell-mediated cytotoxic damage has led to the modern era of immunotherapy in cancer treatment. Agents that release these immune brakes have shown activity to recover dysfunctional T cells and regress various cancer. Both cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and Programmed Death-1 (PD-1) play their role as physiologic brakes on unrestrained cytotoxic T effector function. CTLA-4 (CD 152) is a B7/CD28 family; it mediates immunosuppression by indirectly diminishing signaling through the co-stimulatory receptor CD28. Ipilimumab is the first and only FDA-approved CTLA-4 inhibitor; PD-1 is an inhibitory transmembrane protein expressed on T cells, B cells, Natural Killer cells (NKs), and Myeloid-Derived Suppressor Cells (MDSCs). Programmed Death-Ligand 1 (PD-L1) is expressed on the surface of multiple tissue types, including many tumor cells and hematopoietic cells. PD-L2 is more restricted to hematopoietic cells. Blockade of the PD-1 /PDL-1 pathway can enhance anti-tumor T cell reactivity and promotes immune control over the cancerous cells. Since the FDA approval of ipilimumab (human IgG1 k anti-CTLA-4 monoclonal antibody) in 2011, six more immune checkpoint inhibitors (ICIs) have been approved for cancer therapy. PD-1 inhibitors nivolumab, pembrolizumab, cemiplimab and PD-L1 inhibitors atezolizumab, avelumab, and durvalumab are in the current list of the approved agents in addition to ipilimumab. In this review paper, we discuss the role of each immune checkpoint inhibitor (ICI), the landmark trials which led to their FDA approval, and the strength of the evidence per National Comprehensive Cancer Network (NCCN), which is broadly utilized by medical oncologists and hematologists in their daily practice.
Keywords: CTLA-4; FDA; NCCN; PDL-1; T-cells activation; atezolizumab; avelumab; cancer; cemiplimab; checkpoint inhibitors; durvalumab; immunotherapy; ipilimumab; nivolumab; pembrolizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immune Checkpoint Inhibitors in the Treatment of Cancer.Curr Rev Clin Exp Pharmacol. 2022;17(2):103-113. doi: 10.2174/1574884716666210325095022. Curr Rev Clin Exp Pharmacol. 2022. PMID: 33823768 Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Immune Checkpoint Inhibitors in Cancer Therapy.Curr Oncol. 2022 Apr 24;29(5):3044-3060. doi: 10.3390/curroncol29050247. Curr Oncol. 2022. PMID: 35621637 Free PMC article. Review.
-
Immune checkpoint inhibitors promising role in cancer therapy: clinical evidence and immune-related adverse events.Med Oncol. 2023 Jul 15;40(8):243. doi: 10.1007/s12032-023-02114-6. Med Oncol. 2023. PMID: 37453930 Review.
-
Ileus in patients treated with immune checkpoint inhibitors: A retrospective, pharmacovigilance study using Food and Drug Administration Adverse Event Reporting System database.Pharmacoepidemiol Drug Saf. 2022 Nov;31(11):1199-1205. doi: 10.1002/pds.5493. Epub 2022 Jul 6. Pharmacoepidemiol Drug Saf. 2022. PMID: 35689298
Cited by
-
Immunochemotherapy includes pembrolizumab for stage IIIB lung squamous cell carcinoma: a case report.Ann Transl Med. 2022 Sep;10(18):1028. doi: 10.21037/atm-22-3769. Ann Transl Med. 2022. PMID: 36267777 Free PMC article.
-
Loss of p16: A Bouncer of the Immunological Surveillance?Life (Basel). 2021 Apr 2;11(4):309. doi: 10.3390/life11040309. Life (Basel). 2021. PMID: 33918220 Free PMC article. Review.
-
FDG PET/CT for Assessment of Immune Therapy: Opportunities and Understanding Pitfalls.Semin Nucl Med. 2020 Nov;50(6):518-531. doi: 10.1053/j.semnuclmed.2020.06.001. Epub 2020 Jun 28. Semin Nucl Med. 2020. PMID: 33059821 Free PMC article. Review.
-
Tipping the scales: Immunotherapeutic strategies that disrupt immunosuppression and promote immune activation.Front Immunol. 2022 Sep 8;13:993624. doi: 10.3389/fimmu.2022.993624. eCollection 2022. Front Immunol. 2022. PMID: 36159809 Free PMC article. Review.
-
COMMD1, from the Repair of DNA Double Strand Breaks, to a Novel Anti-Cancer Therapeutic Target.Cancers (Basel). 2021 Feb 16;13(4):830. doi: 10.3390/cancers13040830. Cancers (Basel). 2021. PMID: 33669398 Free PMC article.
References
-
- Murphy S.L., Xu J., Kochanek K.D., Arias E. Mortality in the United States, 2017. Nchs Data Brief. 2018:1–8. - PubMed
-
- French J.D., Kotnis G.R., Said S., Raeburn C.D., McIntyre R.C., Jr., Klopper J.P., Haugen B.R. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2012;97:E934–E943. doi: 10.1210/jc.2011-3428. - DOI - PMC - PubMed
-
- Severson J.J., Serracino H.S., Mateescu V., Raeburn C.D., McIntyre R.C., Jr., Sams S.B., Haugen B.R., French J.D. PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol. Res. 2015;3:620–630. doi: 10.1158/2326-6066.CIR-14-0201. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials